Simple and controllable preparation method of copper-indium-sulfur ternary semiconductor nano granules
A nanoparticle, copper indium sulfur technology, applied in nanotechnology, chemical instruments and methods, gallium/indium/thallium compounds, etc., can solve the problem of inability to control the crystal phase and particle size of CuInS2, complex preparation steps, toxic organic solvents, etc. problem, to achieve the effect of low cost, simple preparation process, and avoid the use of toxic organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 prepares the CuInS of chalcopyrite phase 2 nanoparticles
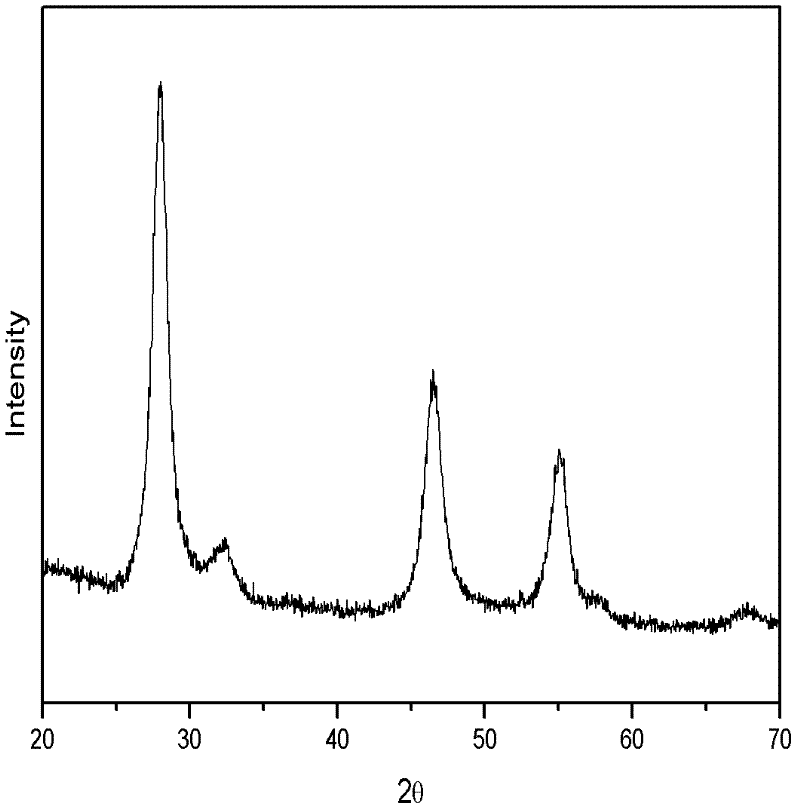
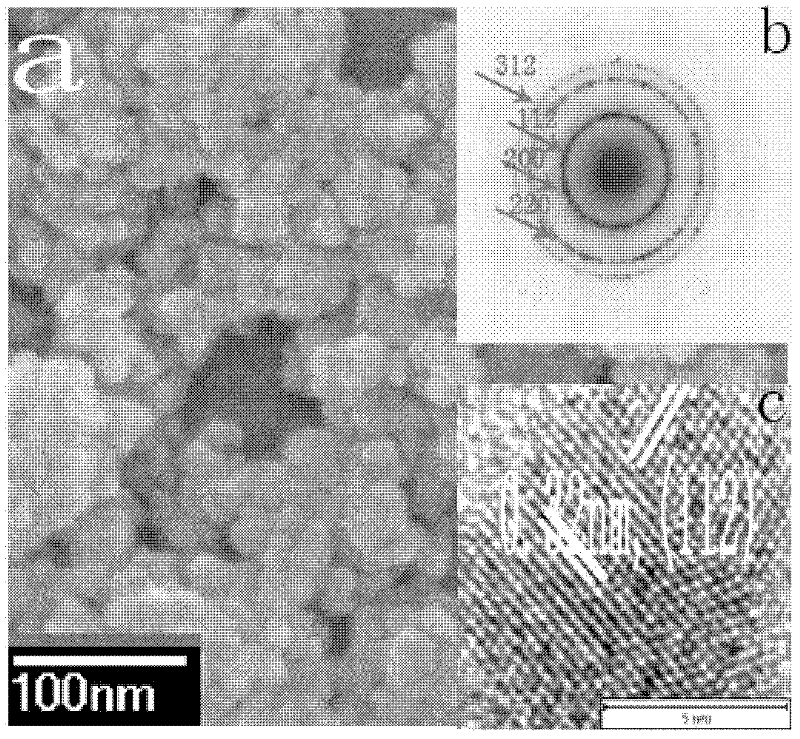
[0048] Dissolve copper nitrate and indium chloride in water with a molar ratio of 1:1, add an appropriate amount of thioglycolic acid to adjust the pH, and then add sodium sulfide, wherein the molar ratio of sodium sulfide to copper sulfate is 2:1. After stirring for a certain period of time, the resulting solution was placed in a reaction kettle and reacted at 160° C. for 20 h. The reactor was naturally cooled to room temperature, centrifugally settled, and then washed repeatedly with deionized water and ethanol, and the obtained nanoparticles were dried at 70°C for 4 hours to obtain a black powder. The X-ray diffraction characterization of the obtained powder sample shows that the obtained copper indium sulfur nanoparticles are chalcopyrite phase, figure 1 Shown is its X-ray diffraction pattern. High-definition transmission electron microscopy and electron diffraction spots further verified tha...
Embodiment 2
[0049] Embodiment 2 prepares the CuInS of wurtzite phase 2 nanoparticles
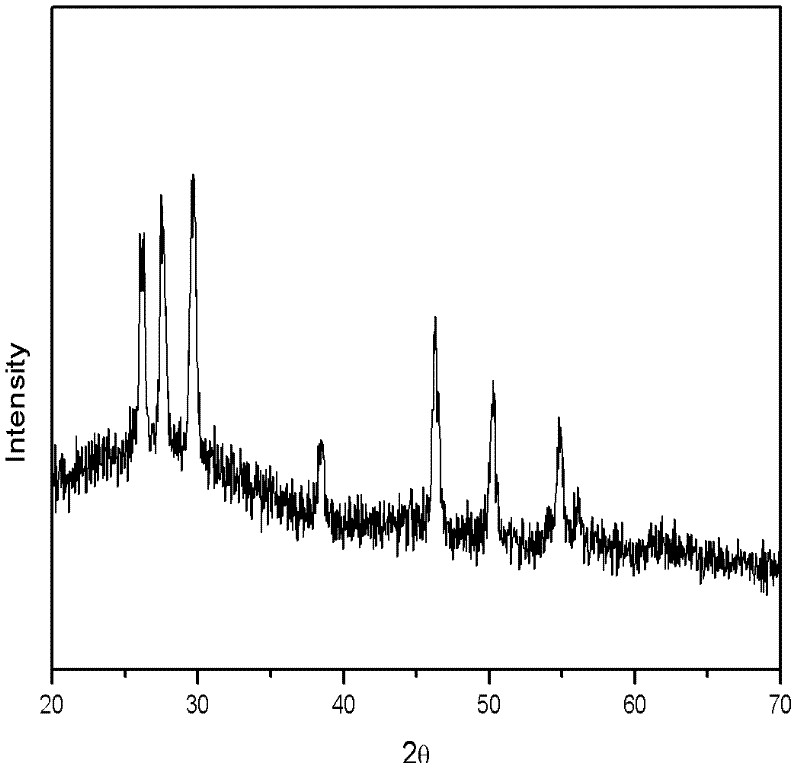
[0050] Dissolve copper sulfate and indium chloride in water at a molar ratio of 1:1, add an appropriate amount of thioglycolic acid, stir for 5 minutes, then add thiourea to adjust the pH, wherein the molar ratio of thiourea to copper sulfate is 2:1. After stirring for a certain period of time, the obtained solution was placed in a reaction kettle and reacted at 140° C. for 15 hours. The reactor was naturally cooled to room temperature, centrifugally settled, and then washed repeatedly with deionized water and ethanol, and the obtained nanoparticles were dried at 70°C for 4 hours to obtain a black powder. The X-ray diffraction characterization of the obtained powder sample shows that the obtained copper indium sulfur nanoparticles are wurtzite phase, image 3 Shown is its X-ray diffraction pattern.
Embodiment 3
[0051] Embodiment 3 prepares the CuInS of wurtzite 2 nanoparticles
[0052] Dissolve copper sulfate and indium chloride in water at a molar ratio of 1:1, add 50ml of mercaptoacetic acid, stir for 5 minutes, then add thiourea to adjust the pH, wherein the molar ratio of thiourea to copper sulfate is 2:1. After stirring for a certain period of time, the resulting solution was placed in a reactor and reacted at 200° C. for 2 hours. The reactor was naturally cooled to room temperature, centrifugally settled, and then washed repeatedly with deionized water and ethanol, and the obtained nanoparticles were dried at 70°C for 4 hours to obtain a black powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com