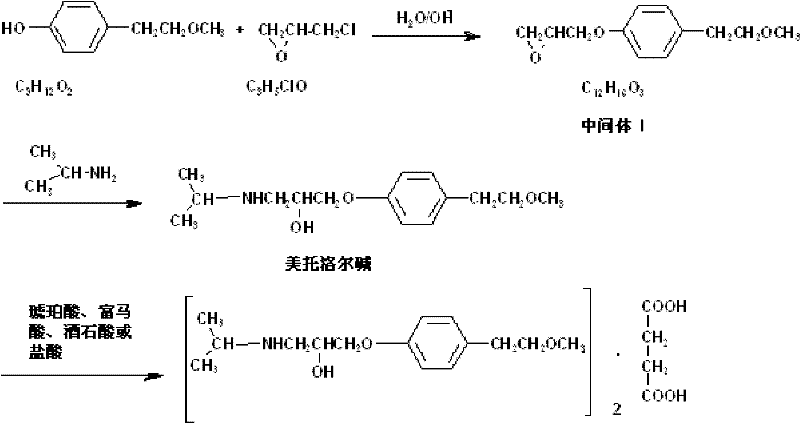
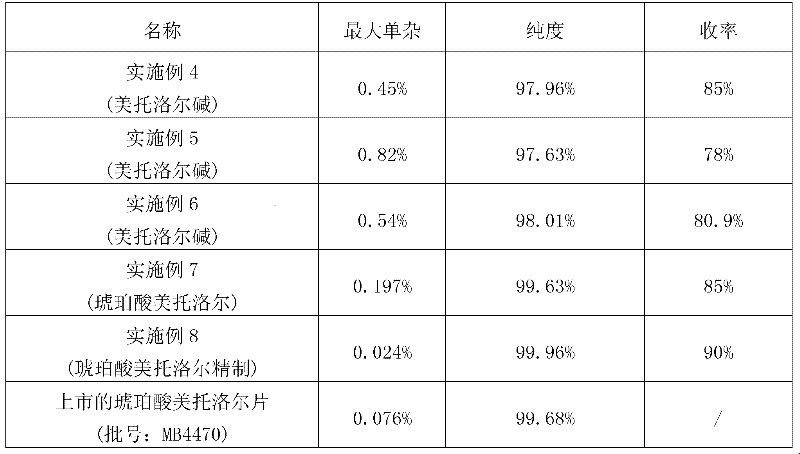
Preparation method for metoprolol salt
A technology of Trolol salt and Trolol base, which is applied in the field of chemistry, can solve the problems of many organic solvents, large environmental pollution, and high cost, and achieve the effects of less organic solvents, less environmental pollution, and a short preparation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1,3-[4-(2-methoxyethyl) phenoxy]-1,2-propylene oxide (intermediate I)
[0033] Add 500ml of purified water to a 3000ml reaction bottle, start stirring, add 304g of p-(2-methoxyethyl)phenol, 158ml of epichlorohydrin (1.0 equivalent), stir for 0.5h until completely dissolved; Sodium 64g (0.8 equivalent), was added to 412ml of purified water to dissolve, after it was completely dissolved, it was lowered to room temperature, and slowly added dropwise to the mixture, the temperature was controlled at 60°C, and the drop rate was controlled, and the dropwise addition was completed in 1.5h; 60 ℃ insulation reaction for 16h. Treatment after completion of the reaction: standing for stratification, retaining the lower organic phase, washing with purified water three times, 600ml of water / time; adding 30g of anhydrous sodium sulfate to dry for 0.5h, filtering, and retaining the filtrate to obtain intermediate I 3-[4-( 400 g of 2-methoxyethyl)phenoxy]-...
Embodiment 2
[0034] Embodiment 2, the preparation of 3-[4-(2-methoxyethyl) phenoxy]-1,2-propylene oxide
[0035] Add 500ml of purified water to a 3000ml reaction flask, start stirring, add 304g of p-(2-methoxyethyl)phenol, 173ml of epichlorohydrin (1.1 equivalent), stir for 0.5h until completely dissolved. Weigh 48g (0.6 equivalent) of sodium hydroxide, add it to 412ml of purified water and dissolve it, cool it down to room temperature after it is completely dissolved, slowly add it dropwise to the mixture, control the temperature at 70°C, and control the rate of addition, and complete the dropwise addition in 1.5h. After the dropwise addition, keep the reaction at 70°C for 16h. Treatment after completion of the reaction: standing for stratification, retaining the lower organic phase, washing with purified water 600ml×3 for three times; adding 30g of anhydrous sodium sulfate to dry for 0.5h, filtering, and retaining the filtrate to obtain intermediate I: 3-[4-(2 -380 g of -methoxyethyl)ph...
Embodiment 3
[0036] Embodiment 3, the preparation of 3-[4-(2-methoxyethyl) phenoxy]-1,2-propylene oxide
[0037] Add 500ml of purified water to a 3000ml reaction flask, start stirring, add 304g of p-(2-methoxyethyl)phenol, 141ml of epichlorohydrin (0.9 equivalent), stir for 0.5h until completely dissolved. Weigh 64g (0.8 equivalent) of sodium hydroxide, add it to 412ml of purified water and dissolve it, cool it down to room temperature after it is completely dissolved, slowly add it dropwise to the mixture, control the temperature at 50°C, and control the rate of addition, and complete the dropwise addition in 1.5 hours. After the dropwise addition was completed, the reaction was incubated at 50° C. for 16 hours. Treatment after completion of the reaction: standing for stratification, retaining the lower organic phase, washing with purified water 600ml×3 for three times; adding 30g of anhydrous sodium sulfate to dry for 0.5h, filtering, and retaining the filtrate to obtain intermediate I: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com