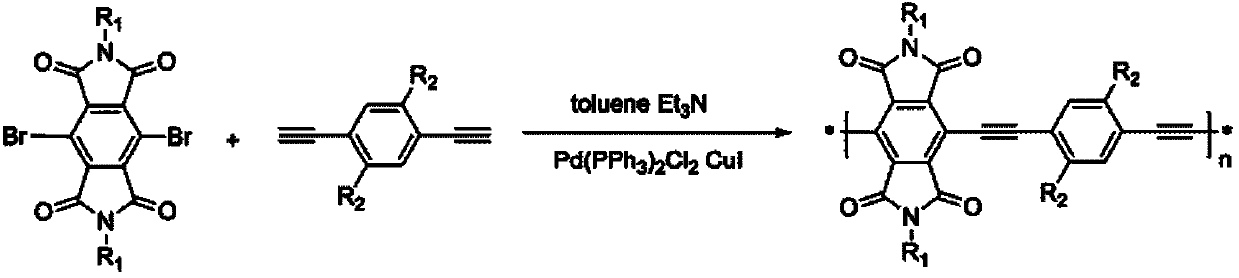
Soluble benzenetetracarboxylic diimide group-containing full-conjugated polymer and preparation method thereof
A tetracarbonyldiimide group, conjugated polymer technology, applied in the field of soluble fully conjugated polymers, to achieve the effect of reducing steric hindrance, easy to arrange regularly, and mild polymerization conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of each monomer is described as follows:
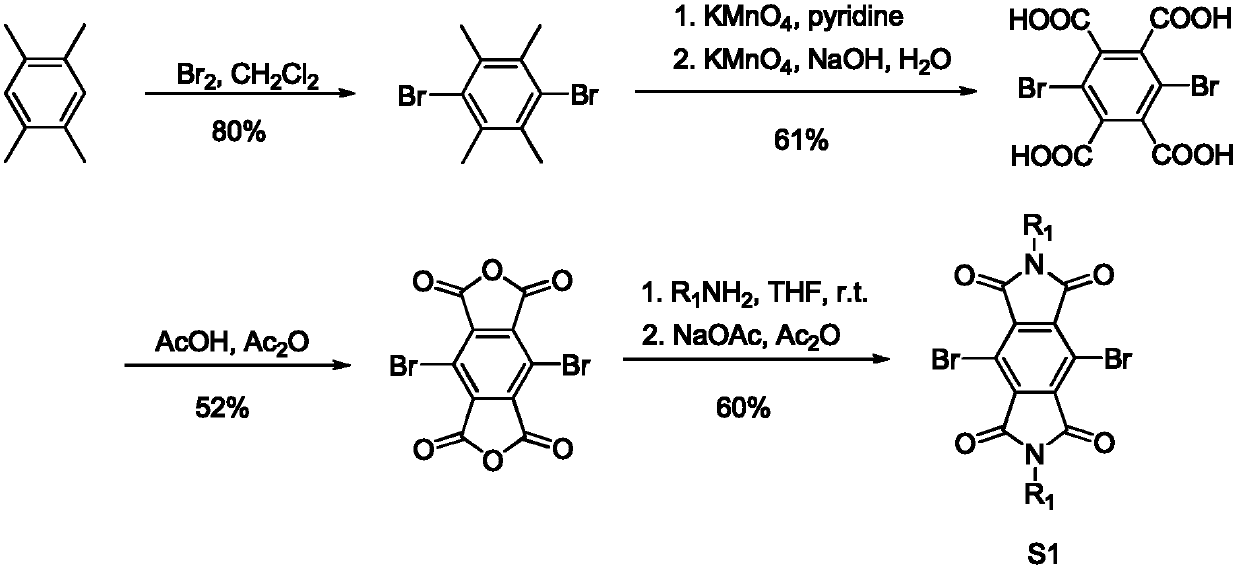
[0027] Preparation of monomer S1 before polymerization
[0028] The schematic diagram of the synthesis route of monomer S1 (dibromobenzenetetracarbonyldiimide and its derivatives) is as follows figure 2 shown. Monomer S1 is prepared by the literature method, and the detailed preparation method can be found in the literature report (J.Org.Chem.2008, 73, 4065-4075.).
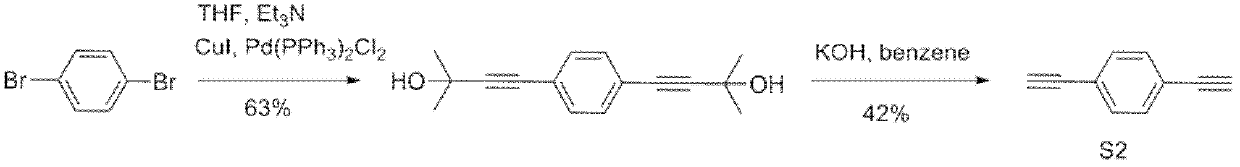
[0029] Preparation of monomer S2 before polymerization
[0030] The schematic diagram of the synthesis route of monomer S2 (1,4 diynylbenzene) is as follows image 3 shown. Monomer S2 is prepared by using p-dibromobenzene as a raw material by a literature method, and the detailed preparation method can be found in literature reports (Synthetic Communications, 1996, 26, 2309-2316).
[0031] Preparation of monomer S3 before polymerization
[0032] The schematic diagram of the synthetic route of monomer S3 (p-2-alkynyl (2,5-trifluor...
Embodiment 1
[0033] Embodiment 1, synthetic polymer P1
[0034] The schematic diagram of the synthetic route of polymer P1 is as follows: Figure 5 As shown, the specific steps are: add 1mmol monomer S1 into a 100mL glass reaction bottle (at this time, R1 corresponding to monomer S1 is as shown in Table 1, which is -CH 2 CH(C 8 h 17 )C 10 h 21 ) and 1mmol monomer S2, then add 15mL dry toluene and 10mL dry triethylamine, stir and add 0.05mmol ditriphenylphosphine palladium dichloride and 0.1mmol cuprous iodide, react at 60°C for 24 hours Stop the reaction, pour into 200mL methanol for precipitation, and filter with suction to obtain a solid. The polymer was sequentially extracted with methanol, acetone and n-hexane Soxhlet, finally dissolved with chloroform, and finally precipitated with methanol to obtain the orange polymer P1, whose structural formula is shown in Table 1. Electrochemical test graph of polymer P1 as Figure 6 As shown, it can be seen from the figure that the start...
Embodiment 2~3
[0035] Embodiment 2~3, synthetic polymer P2~P3
[0036] The specific steps are the same as in Example 1: add 1 mmol of monomer S1 into a 100mL glass reaction bottle (at this time, R1 corresponding to monomer S1 is shown in Table 1, which are: -CH 2 CH(C 2 h 5 )C 4 h 9 ,-C 12 h 25 ) and 1mmol monomer S2, then add 15mL dry toluene and 10mL dry triethylamine, stir and add 0.05mmol ditriphenylphosphine palladium dichloride and 0.1mmol cuprous iodide, and react at 30°C for 2 hours Stop the reaction, pour into 200mL methanol for precipitation, and filter with suction to obtain a solid. The polymers were sequentially extracted with methanol, acetone and n-hexane Soxhlet, and finally dissolved in chloroform, and finally precipitated with methanol to obtain orange polymers P2-P3, whose structural formulas are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com