CEA TRFIA (time-resolved fluoroimmunoassay) kit based on IMB (immunomagnetic beads)
A technology of time-resolved fluorescence and immunomagnetic beads, which is applied in the fields of nanobiology and bioanalytical chemistry, can solve the problems that tumor-associated antigens have not been reported in the literature, and achieve the effects of shortening the reaction time, widening the range of the standard curve, and improving the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Preparation of the kit of the present invention
[0037] (1) Specific steps for the preparation of immunomagnetic beads:
[0038]The first step, pretreatment of magnetic beads: use a vortex mixer to fully mix and suspend magnetic beads (Merck company product, number: 39572001), take 1ml (10mg) of the suspension in a centrifuge tube, and place it on a magnetic separator 1min (or longer), carefully remove the supernatant. Add 1ml Binding Buffer, mix thoroughly with a vortex mixer to suspend the magnetic beads, and place on a magnetic separator for 1min (or longer) to carefully remove the supernatant, repeat this step twice, then add 25 μl EDC (10mg / ml) and 40 μl NHS (10mg / ml), use a sample rotary mixer and rotate at room temperature for 30min. Add 1ml Binding Buffer, mix well with a vortex mixer to suspend the magnetic beads, and place on a magnetic separator for 1min (or longer) to carefully remove the supernatant, repeat this step 2 times.
[0039] The ...
Embodiment 2
[0060] Embodiment 2 The usage method of the kit of the present invention
[0061] (1) Sample collection
[0062] Take 1-2ml of venous blood into the coagulation tube, place it at 4°C for more than 2 hours, and take 25ml of serum after the serum is precipitated. Serum samples can be stored at 2-8°C for 7 days. If long-term storage is required, please store at -20°C to avoid repeated freezing and thawing. Samples need to be transported in vacuum flasks or other devices containing dry ice.
[0063] (2) Preparation of reagents
[0064] 1) Washing solution: Mix 50 mL of concentrated washing solution and 1200 mL of deionized water as a working washing solution.
[0065] 2) Marker working solution: One hour before use, dissolve each bottle of marker with 1 mL of deionized water, and dilute it 1:50 times with analysis buffer as the europium-labeled antibody working solution.
[0066] 3) Immunomagnetic beads: shake and suspend before use.
[0067] (3) Operation steps
[0068] 1) ...
Embodiment 3
[0074] Embodiment 3 The methodological test of the kit of the present invention
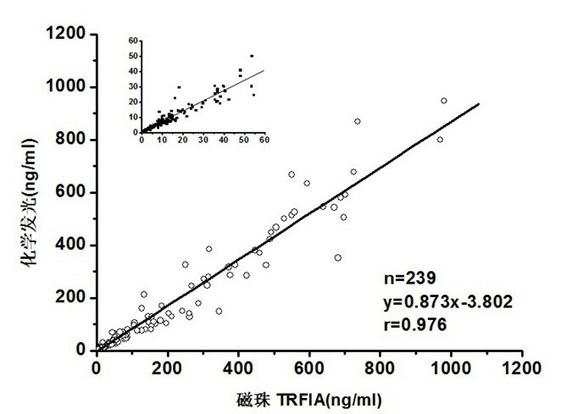
[0075] The test kit prepared in Example 1 is tested according to the routine manufacturing and testing procedures in the art, and the results are as follows:
[0076] 1) Accuracy
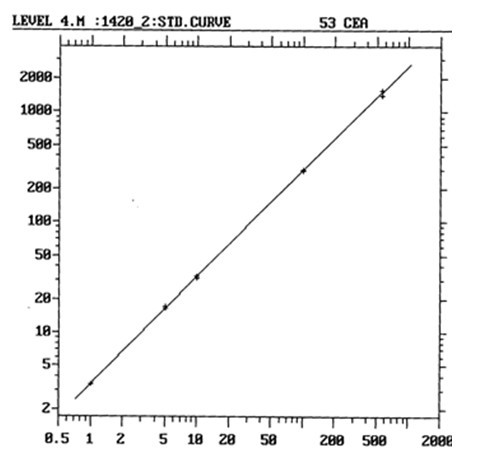
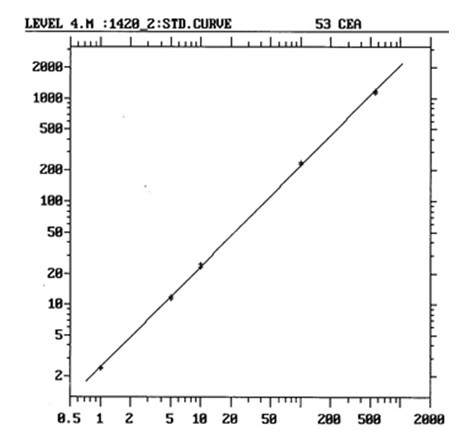
[0077] After analyzing and measuring the calibrator and the corresponding national standard substance at the same time, the two dose-response curves are basically parallel ( figure 1 : is the dose-response curve of the national standard; figure 2 : is the dose-response curve of the calibrator; Cps is the fluorescence count per second), the slopes of the two curves are 0.461 and 0.458 ( t test P >0.05). With the CEA national standard as the reference substance, the ratio of the measured titer to the marked potency of the calibrator is between 0.9 and 1.1. The linear correlation coefficient of the dose-response curve ( r )=0.998.
[0078] 2) Analytical sensitivity and linear range
[0079] The zero reference sta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com