Aromatic thiazole compounds, analogues thereof, uses thereof and preparation methods thereof
A technology based on thiazoles and organic compounds, which is applied in the field of preparation of aryl thiazoles and their analogs, can solve problems that have not been reported in relevant literature, and achieve remarkable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0113] Example 1-1, 2-(2-ethyl-4-pyridyl)-4-methylthiazole-5-carboxylic acid ethyl ester (KZW001)
[0114] Dissolve the compound 2-ethylpyridyl 4-sulfuramide (332mg, 2.0mmol) in absolute ethanol (5ml), add ethyl bromoacetoacetate (627mg, 3.0mmol) to the reaction system under a nitrogen atmosphere Heat to reflux, stop the reaction after TLC detects that there is no raw material, cool to room temperature, remove the solvent under reduced pressure, and obtain compound 2-(2-ethyl-4-pyridyl)-4-methylthiazole after purification by column chromatography -Ethyl 5-carboxylate (475 mg, 86%): 1 H NMR (300MHz, CDCl 3 ): 8.63(d, J=5.1Hz, 1H), 7.70(s, 1H), 7.60(d, J=5.1Hz, 1H), 4.37(q, J=7.2Hz, 2H), 2.91(q, J =7.5Hz, 2H), 2.80(s, 3H), 1.40(t, J=7.2Hz, 3H), 1.36(t, J=7.5Hz, 3H).
Embodiment 1-2 to 1-35
[0115] Preparation of KZW002-45 carboline compounds shown in Examples 1-2 to 1-35 and Table 1 (see reference below for specific process)
[0116]
[0117]
[0118]
[0119]
[0120]
[0121] Table 1
Embodiment 2
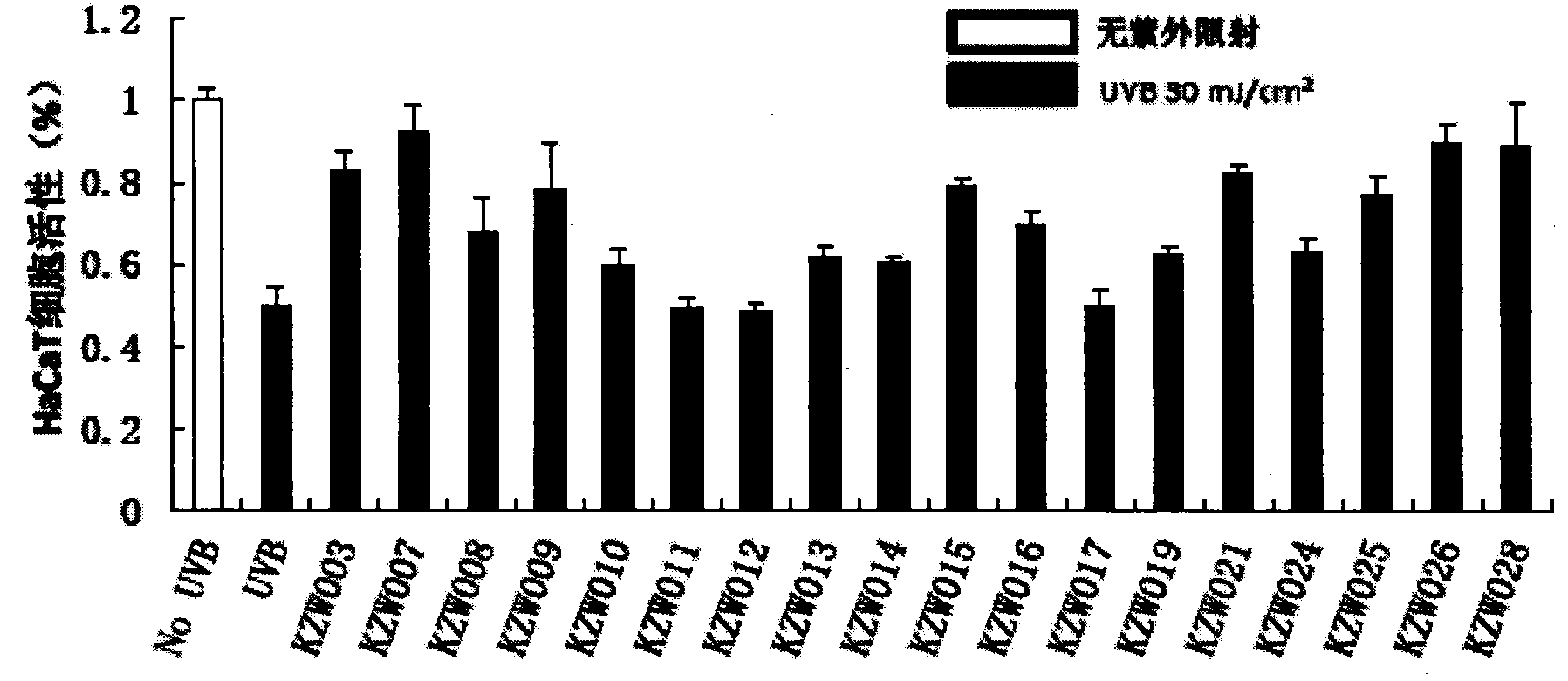
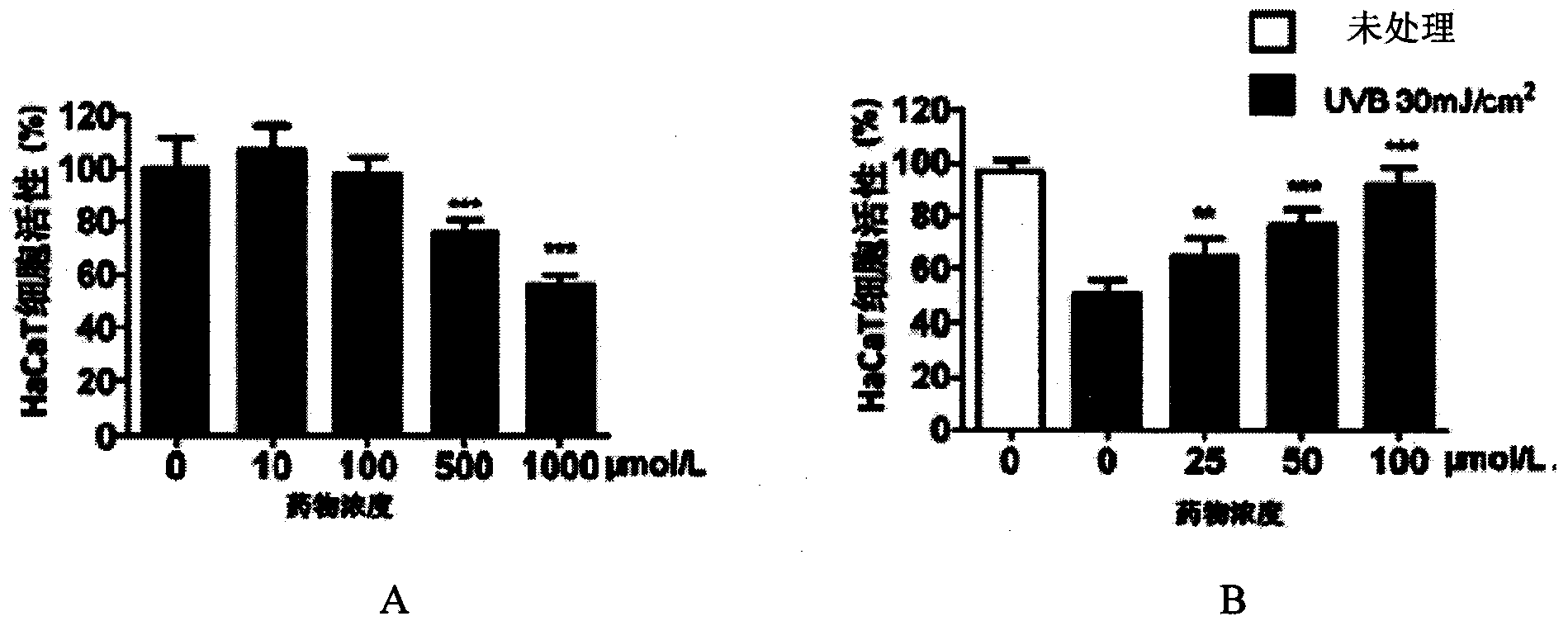
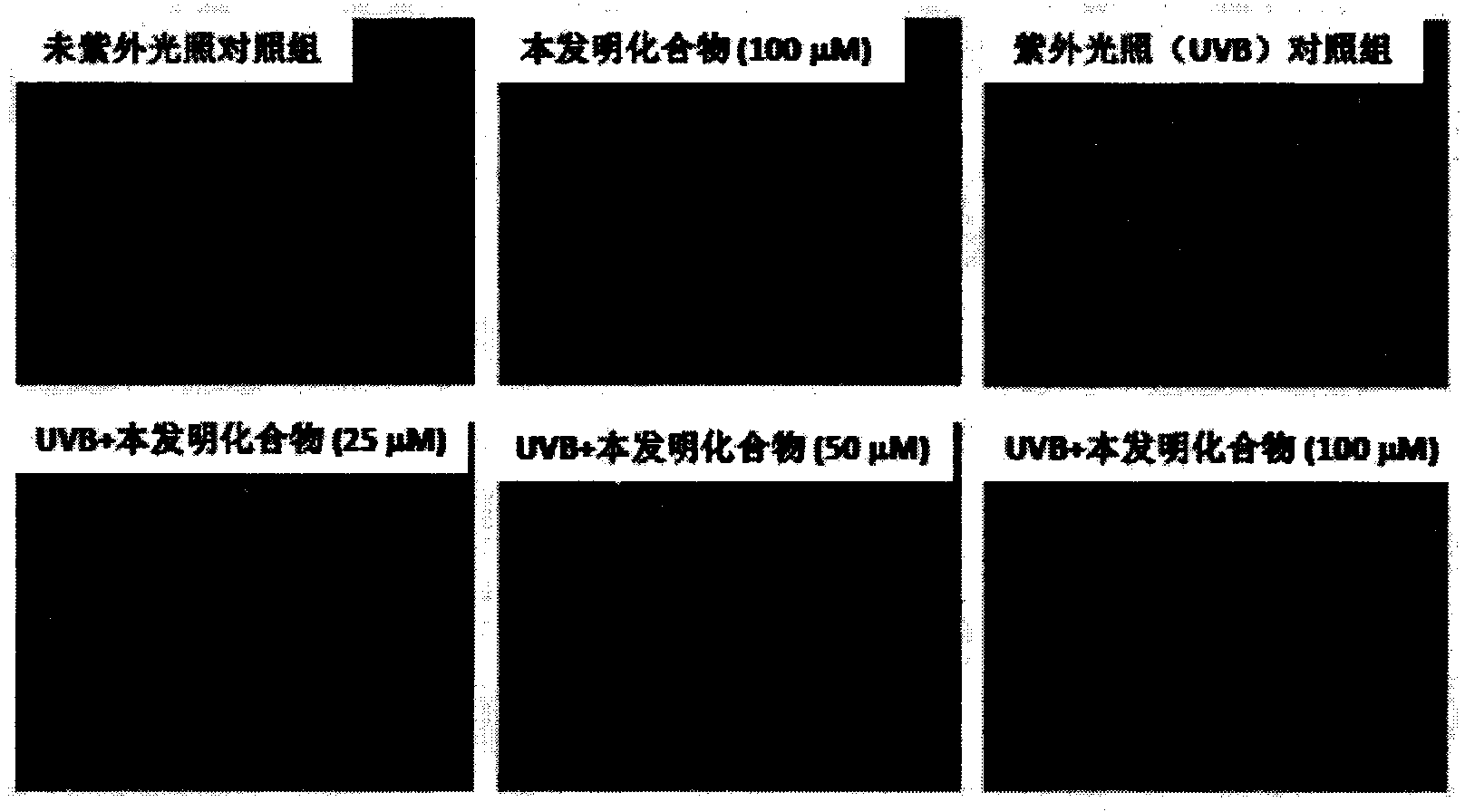
[0122] Example 2: The protective effect of a certain dose of the compound of the present invention on the skin under ultraviolet irradiation and the inhibition of apoptosis and the increase of COX2 expression
[0123] 2-1. Cell culture
[0124] Human normal skin HaCaT cells used in this experiment were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences, and cultured in a 37°C constant temperature incubator (humidity 95%, CO 2 5%), cultured in RPMI-1640 medium containing 100U / mL penicillin, 100μg / mL streptomycin and 10% calf serum, changing the medium every day, and passaged once every 2-3 days. Inoculated in 90mm Petri dishes.
[0125] 2-2, the protective effect of the compound of the present invention on ultraviolet UVB radiation HaCaT cells
[0126] Human skin keratinocytes HaCaT were treated with 1 × 10 5 The cell density of cells / ml was placed in a 96-well plate. After 12 hours of conventional culture, the compound of the present invention was adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com