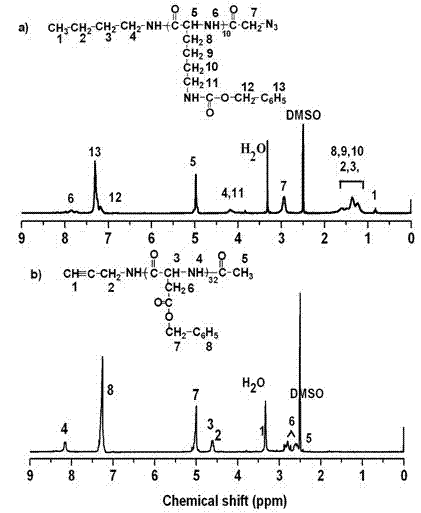
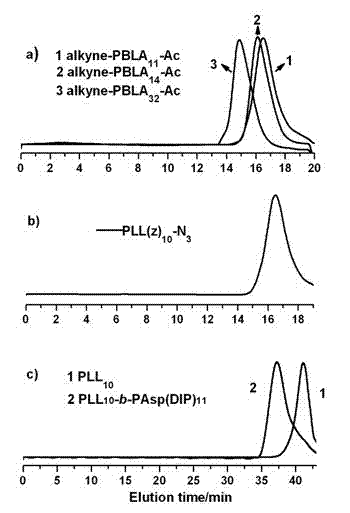
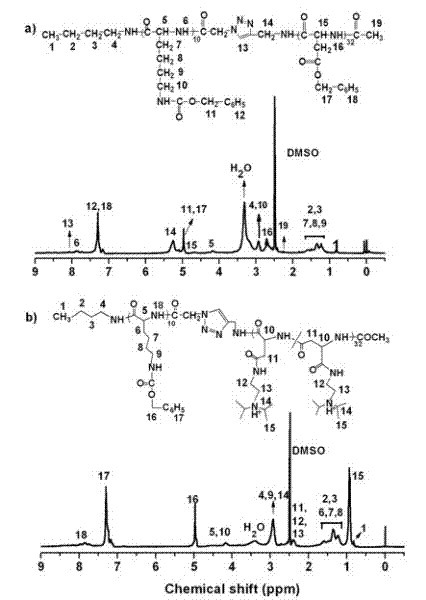
Degradable acid-sensitive macromolecular amphipathic cationic block copolymers and micellar particles and preparation method thereof
A block copolymer, cationic technology, applied in the fields of pharmacy, biomedical engineering, and polymer chemistry, to achieve good synergy, excellent biodegradability, and promote progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of Biodegradable Acid Sensitive Amphiphilic Cationic Block Copolymers
[0039] 1. Preparation of amphiphilic block copolymers:
[0040] 1.1 Preparation of L-type amino acid benzyl ester N-carboxylic acid anhydride Take benzyl aspartate N-carboxylic acid anhydride as an example (BLA-NCA):
[0041] 25.0 g (0.11 mol) benzyl β-aspartate was suspended in 150 mL of dry THF, under argon protection, and heated to 40 °C. 13.5 g (0.045 mol) trimeric phosgene was dissolved in 25 mL of THF Then slowly add it dropwise into the reaction flask. After the reaction solution became clear, the reaction was continued for another 1 hour. Argon flow was bubbled for 30 minutes to remove the generated HCl. The reaction solution was filtered to remove a small amount of unreacted benzyl ester, then concentrated under reduced pressure to 50 mL, precipitated into a large amount of n-hexane (THF / hexane: 1 / 3), and recrystallized the initial product BLA-NCA in THF / hexane mixed solven...
Embodiment 2
[0056] Preparation of polymeric nanocarriers
[0057] 2.1 PLL(z) 10 - b -PAsp(DIP) 32 - Preparation of Ac polymer blank micelles
[0058] Dissolve 10 mg of polymer in 1 ml of DMSO / THF mixed solvent, stir thoroughly, add 0.5 mL of triethylamine to deprotonate the primary amino groups in the polylysine block, slowly drop to 10 mL of pH 7.4 In PBS solution. The micellar solution was dialyzed against PBS for 12 h and freeze-dried. Filter through a 0.45 μm aqueous phase filter, concentrate in an ultrafiltration centrifuge tube to 10 mL, that is, the polymer concentration is 1 mg / mL, and store in a refrigerator at 4 °C for later use.
[0059] 2.2 Loaded DOX polymer PLL(z) 10 - b -P Asp(DIP) 32 - Preparation of Ac micelles
[0060]2 mg water-soluble DOX was dissolved in 1 mL DMSO / THF mixed solvent, and 0.5 mL triethylamine was added to make DOX deprotonated from hydrophilic to hydrophobic; 10 mg polymer was dissolved in 1 mL DMSO / THF mixed solvent , add 0.5 mL triethylamine...
Embodiment 3
[0064] 3.1 Measurement of nanometer drug-loaded micelles particle size
[0065] The resulting 1 mg / mL polymer blank micelle solution was measured by dynamic light scattering method for its particle size PLL 34 - b -PAsp(DIP) 12 and PLL 10 - b -PAsp(DIP) 11 The average particle diameters of the prepared micelles were 38.0 nm and 40.0 nm, respectively.
[0066] 3.2 Determination of critical micelle concentration
[0067] Prepare pyrene into 3.0×10-4 M benzene solution, weigh a certain amount of polymer after the benzene volatilizes, use pH 7.4 PBS solution to constant volume, and then prepare 0.1-1000 ug / ml polymer and pyrene aqueous solution. Each sample was sonicated for 30 minutes at low intensity to promote the inclusion of pyrene into hydrophobic micelles, and then its fluorescence intensity was detected.
[0068] 3.3 Measurement of fluorescence spectrum
[0069] Polymer PLL(z) that will load DOX 10 - b -PAsp(DIP) 32 -Ac micellar solutions were adjusted to pH 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com