Polymerization fluorescent dye, preparation method and application thereof
A fluorescent dye and dye technology, applied in anthracene dyes, luminescent materials, organic dyes, etc., can solve the problems of slow polymerization process and few systems, and achieve the effect of high photostability and high fluorescence quantum yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of polymerizable fluorescent dye 3:
[0042]
[0043] Synthesis of intermediate 2: 4-bromo-N-methylthrapyridone (1, 4.9g, 14.4mmol), anhydrous copper sulfate (0.2g, 10mol%) and 1,6-hexanediamine (2g, 17.2mmol) was dissolved in 100mL ethylene glycol monomethyl ether, and anhydrous potassium carbonate (3g, 21.6mmol) was added as an acid-binding agent. After reflux for 24 hours, potassium carbonate and copper sulfate were removed by filtration. The filtrate was freed of solvent using rotary evaporation to give a red solid. The solid was washed with ethanol to remove excess 1,6-hexanediamine. Silica gel column separation (ethyl acetate:methanol=10:1 as eluent), the yield was 51%.
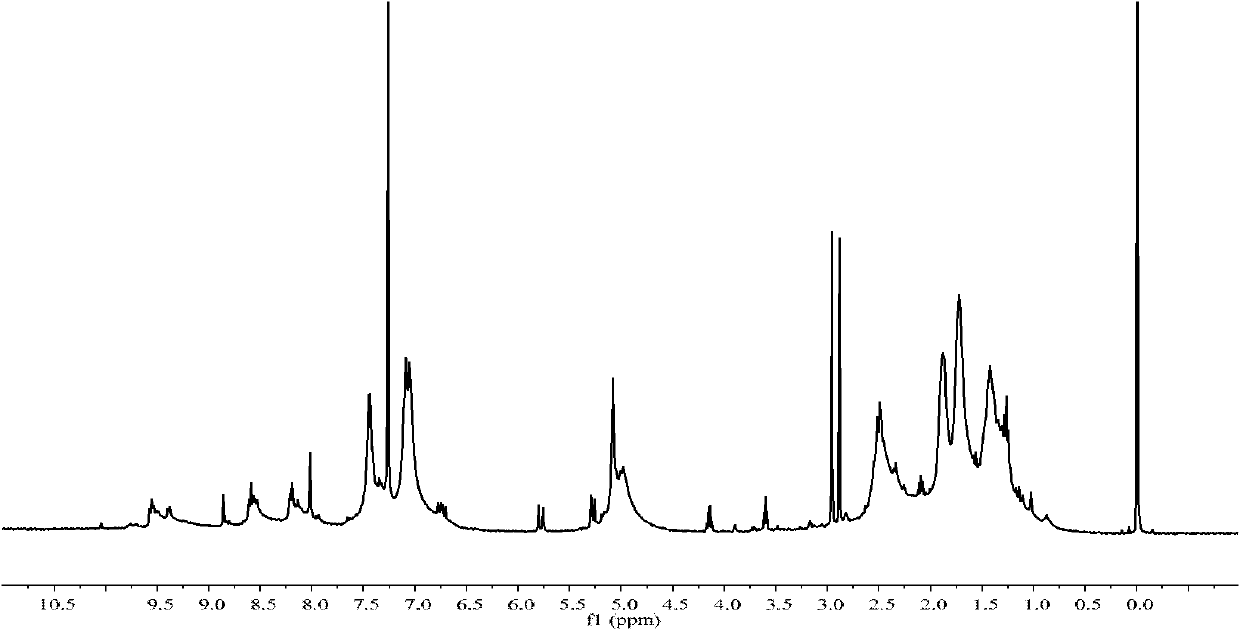
[0044] 1 H-NMR (400MHz, CDCl 3 )δ10.49(m, 1H, NH), 8.46(d, J=7.7Hz, 1H, ArH,), 8.30(d, J=7.9Hz, 1H, ArH), 8.20(d, J=7.9Hz, 1H, ArH), 7.83-7.62 (m, 4H, ArH, CH 2 ), 3.86(s, 3H, CH3), 1.71-1.60(nb, 12H, CH 2 ).MS(TOF MS ES+) calculated for [C 23 h 26 N 3 o 2 ] + : 376.2020...
Embodiment 2
[0048] Synthesis of polymerizable fluorescent dye 5:
[0049] Polymerizable naphthalimide fluorescent dyes were prepared from 4-bromo-1,8-naphthalene anhydride as starting material.
[0050]
[0051] Synthesis of intermediate 4: 4-bromo-1,8-naphthalene anhydride (5.5g, 19.9mmol) and 1,6-hexanediamine (4.6g, 39.7mmol) were dissolved in 200mL ethylene glycol monomethyl ether, reflux After 5h, a large amount of yellow solid precipitated out. After the reaction, the reaction solution was poured into 500 mL of water, filtered, and the filter cake was washed with cold ethanol to remove residual 1,6-hexanediamine. After drying, 5.1 g of yellow solid was obtained with a yield of 63%, which was used for the synthesis of dye 5 without further purification.
[0052] Dye 5 can be obtained by amidation of intermediate 4, and the reaction steps refer to the process of synthesis of dye 3.
[0053] 1 H-NMR (400MHz, CDCl 3 )δ8.57(d, 1H, J=4.0, ArH), 8.45(d, 1H, J=8.0, ArH), 8.29(d, 1H,...
Embodiment 3
[0055] Synthesis of polymerizable fluorescent dye 8:
[0056]
[0057] Synthesis of Intermediate 6: 4-bromo-1,8-naphthalene anhydride (2.7g, 10mmol) and 3-octylamine (1.5g, 12mmol) were dissolved in 100mL ethanol solution, heated to reflux for 10h, no raw material was detected by TLC 4-Bromo-1,8-naphthalene anhydride was cooled to room temperature, and a large amount of khaki solids precipitated out. The reaction solution was filtered, and the filter cake was washed with a small amount of cold ethanol solution until the filtrate had no yellow color. After drying, 3.0 g of an earthy yellow product was obtained, with a crude yield of 88%.
[0058] Synthesis of Intermediate 7: Intermediate 6 (3.0g, 7.7mmol) and anhydrous piperazine (5.0g, 58mmol) were dissolved in 100mL of ethylene glycol monomethyl ether, heated to 100°C, the reaction solution turned yellowish brown, After continuing to react for 6h, it was cooled to room temperature. The reaction solution was poured into 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com