Telmisartan medicinal composition, telmisartan medicinal composition tablets and preparation method for telmisartan medicinal composition tablets
A technology of telmisartan and medicine, which is applied in the field of pharmaceutical preparations and its preparation, can solve the problems affecting the quality of telmisartan sodium salt, telmisartan is difficult to dissolve in water, and low bioavailability, and achieve water solubility Good, less side effects, kidney protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 The preparation method of telmisartan sodium salt
[0018] Utilizing the acidic nature of the carboxyl group in the telmisartan structure, it is refined by alkali-dissolving and acid-precipitating methods. Dissolve telmisartan in ammonia water first, then add activated carbon to decolorize, filter, add hydrochloric acid dropwise to the filtrate to adjust pH, and crystallize to obtain telmisartan fine product. Adopting this refining process route does not use organic solvents chloroform and DMF, and eliminates the distillation operation, the operation is simple and economical, and there is no organic solvent residue.
[0019] Under the condition of lower than 15°C, weigh 0.02 mol of fine telmisartan about 10.3 g, and dissolve the pure telmisartan with 1-5 mol / L ammonia water to obtain telmisartan ammonium salt solution;
[0020] Weigh NaOH 0.022mol, about 0.88g, dissolve NaOH with 40-60% ethanol to obtain NaOH ethanol solution, drop NaOH ethanol solution ...
Embodiment 2
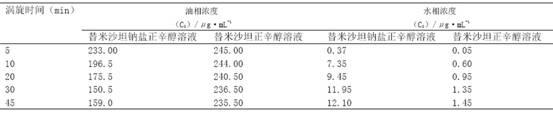
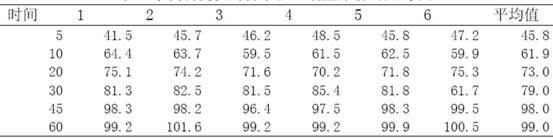
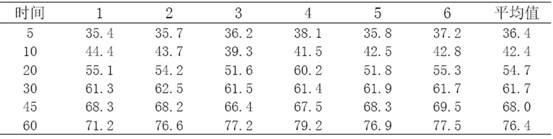
[0022] The investigation of the oil-water partition coefficient of embodiment 2 telmisartan sodium salt
[0023] Accurately weigh 2.5 mg of telmisartan, approximately 0.00000486 moL, dissolve it in n-octanol, and dilute to 10 mL to obtain a 250 μg / mL telmisartan n-octanol solution. Precisely pipette an appropriate amount of the above solution and dilute it with n-octanol to obtain 100 mu g / mL telmisartan n-octanol solution.
[0024] Accurately take by weighing 2.7 mg of telmisartan sodium salt prepared in Example 1, wherein the content of telmisartan sodium salt is about 0.00000486moL, and the operation is as preparing telmisartan n-octanol solution to obtain telmisartan sodium salt n-octanol octanol solution.
[0025] According to the appendix of Chinese Pharmacopoeia 2010 edition, prepare phosphate buffer solution. After measurement, the pH of the solution is 7.29; prepare 0.1mol / L hydrochloric acid solution according to the appendix of Chinese Pharmacopoeia 2010 editio...
Embodiment 3
[0033] Embodiment 3 The preparation method of telmisartan sodium salt tablet
[0034] The telmisartan sodium salt particles were mixed and ground for more than 60 minutes by adding lactose in increments, then mixed with microcrystalline cellulose and sorbitol and ground again for more than 20 minutes, and the grinding temperature was lower than 30°C to obtain a telmisartan lactose mixture;
[0035]Add polacrilin potassium and anhydrous calcium phosphate to the telmisartan sodium salt mixture, put it into a wet granulator and mix for more than 3 minutes, take Tween-80 and dissolve it in 95% ethanol according to the ratio of 2-4ml per kilogram of preparation weight , and then mixed with 0.5% povidone K30 aqueous solution at a ratio of 1:20 as a binder and added to the wet granulator mixture for granulation.
[0036] The granules in the above-mentioned addition are mixed and added with magnesium stearate, the hardness of the tablet machine is controlled within the 4-5 kgf metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com