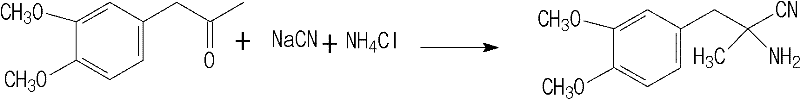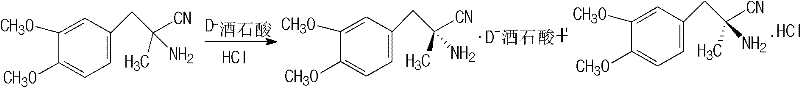Preparation method of L-methyldopa
A technology of L-methyldopa and racemization, which is applied in the field of preparation of L-methyldopa, can solve the problems of polluting the environment, increasing the cost of three wastes, and no literature reports about it, and achieves simple treatment methods and molar total Increased yield and good environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1) Synthesis of DL-aminopropionitrile
[0055] Dissolve 515g (3.15mol) of 30% sodium cyanide aqueous solution and 163g (3.05mol) of ammonium chloride in 2200ml (23.3mol) of 10.6mol / L ammonia solution at room temperature, heat to 55°C, and rapidly stir Add 582.6g (3mol) veratrone, stir for another 1h, then cool down to 20°C-25°C in 1-1.5h, the product with fine particles begins to precipitate, keep stirring for 2h, cool down to 0°C and filter with suction, the filtrate is DL- The mother liquor of aminopropionitrile synthesis (2089ml), the filter cake was washed with 800ml of ice water, and dried to obtain 623.17g of DL-aminopropionitrile (94.3% molar yield), with a melting point of 85°C to 87°C. The obtained DL-aminopropionitrile can be directly resolved without purification.
[0056] 154.5g (3.15mol) 96%~98% sodium cyanide and 160.5g (3mol) ammonium chloride room temperature are dissolved in the mother liquor that the DL-aminopropionitrile that the last step of 2089ml ...
Embodiment 2
[0076] Add 2089ml of DL-aminopropionitrile synthesis mother liquor (COD: 48271ppm, pH=10.5) obtained in step 1) of Example 1 to the reaction flask, add 600ml of dichloroethane, stir and heat up to reflux, reflux for 5h, After the reaction was completed, cool to room temperature, let stand to separate layers, and extract the water layer with 300ml dichloroethane again to obtain a water layer and an organic layer;
[0077] COD detected in the water layer: 13567ppm, most of which are caused by dichloroethane dissolved in water. After aeration for 5 hours, the detected COD: 4368ppm, the COD removal rate is 91%. The COD is passed through the COD rapid detector, using the national general standard;
[0078] The organic layers were combined, after the solvent was recovered by distillation, high-vacuum distillation (the pressure of high-vacuum distillation was 2mmHg, 0.266kPa), and 28.5g of fractions at 112°C to 120°C were collected, and the content of veratrone obtained by gas chromat...
Embodiment 3
[0080] Add 1500ml of DL-aminopropionitrile synthesis mother liquor (COD: 46638ppm, pH=10.3) obtained in step 1) of Comparative Example 1 to the reaction flask, add 300ml of dichloromethane, stir and heat up to reflux, reflux for 12h, cool after the reaction is completed to room temperature, standing and stratifying to obtain an aqueous layer and an organic layer;
[0081] COD detected in the water layer: 14639ppm, most of which are caused by the dissolution of dichloromethane in water. After aeration for 5 hours, the detected COD: 4691ppm, and the COD removal rate is 90%. The COD is passed through the COD rapid detector, using the national general standard;
[0082] After the organic layer was distilled to recover the solvent, high vacuum distillation (the pressure of high vacuum distillation was 2mmHg, 0.266kPa), collected 20.2g of cuts at 112°C to 120°C, and gas chromatography (GC) detected that the content of veratrone was 98.86%. The rutrune content was obtained by the are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com