Method for preparing cecropins AD and frog Buforin II fusion antimicrobial peptide by using hydroxylamine cutting method, and uses thereof
A technology of hydroxylamine cutting and cecropin, applied in the field of bioengineering, can solve the problems of affecting the activity of antibacterial peptides, low expression efficiency, long growth cycle of yeast, etc., and achieve the effects of reducing production cost and simplifying purification process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Synthesis of cecropin AD and frog Buforin II fusion gene CAD-Buforin II
[0038] 1 Synthesize the fusion gene of Cecropin AD and Frog Buforin II, design 4 pairs of primers:
[0039] F1
[0040] GGATCC AAATGGAAACTGTTCAAAAAAATCGAAAAAGTTGGTCAGCGTGTTCGT
[0041] R1
[0042] AGCAGAGATAACAGCGTCACGAACACGCTGACCAACTTTTTCGA
[0043] F2
[0044] GACGCTGTTATCTCTGCTGGTCCGGGTGTTGCTACCTTCG
[0045] R2
[0046] TTAGCCAGAGCGGTAGCCTGAGCGAAGGTAGCAACACCCGGACC
[0047] F3
[0048] CTCAGGCTACCGCTCTGGCTAAA ACCCGTTCTTCTCGTGCT
[0049] R3
[0050] ACCAACCGGGAACTGCAGACCAGCACGAGAAGAACGGGT T
[0051] F4
[0052] GGTCTGCAGTTCCCGGTTGGTCGTGTTCACCGTCTGCTGCGTAAAGAATTC
[0053] R4
[0054] GAATTC TTTACGCAGCAGACGGTGAACACG
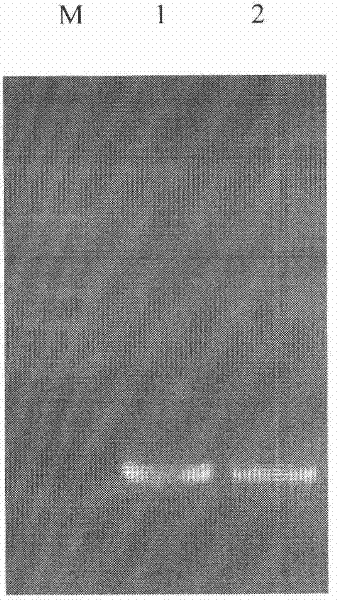
[0055] The fusion gene CAD-Buforin II gene of Cecropin AD and Frog Buforin II was synthesized by 3 rounds of PCR. The 5'end and 3'end of the fusion gene were added with BamH I and EcoR I restriction enzyme sites (underlined Out), a hydroxylamine cleavage site (in bold) is added to the 5'end...
Embodiment 2
[0064] Example 2 Construction of CAD-Buforin II gene cloning and expression recombinant plasmid
[0065] 2.1 The construction of CAD-Buforin II gene recombinant plasmid:
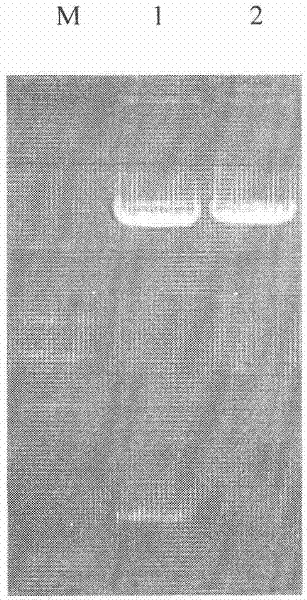
[0066] The plasmid pET-Trx was extracted and digested with BamH I and EcoR I to recover a 3.3 kb linear plasmid;
[0067] The artificially synthesized CAD-Buforin II gene fragment in step 1 was digested with BamH I and EcoR I, and an equal volume of phenol: chloroform: isoamyl alcohol (volume ratio 25: 24:1) was added to extract, 3 times the volume Absolute ethanol precipitation to recover the digested fragments;
[0068] The fragments of the digested product recovered above were ligated with T4DNA ligase, and the ligation reaction system was 25μl:
[0069]
[0070] The ligation was carried out overnight at 16°C, and the ligation product was the pET-Trx-CAD-Buforin II recombinant plasmid.
[0071] 2.2 The recombinant plasmid pET-Trx-CAD-Buforin II was transformed into E. coli Top 10 to verify the recombination results...
Embodiment 3
[0073] Example 3 Construction and screening of engineering strains expressing CAD-Buforin II
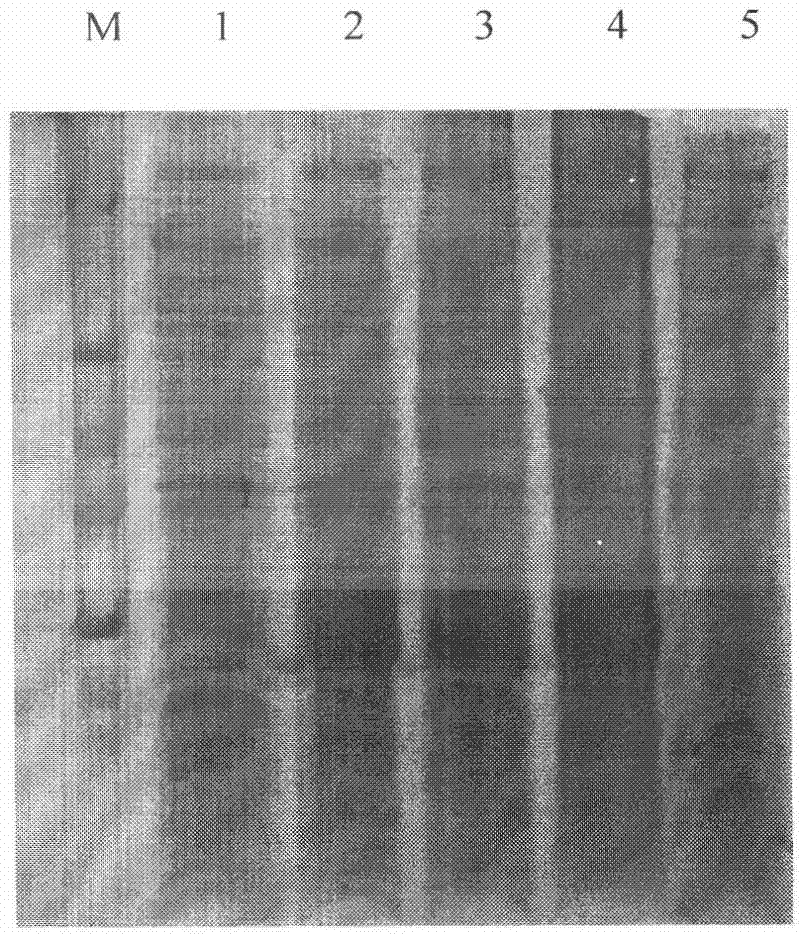
[0074] 3.1 The recombinant plasmid pET-Trx-CAD-Buforin II transforms the expression strain BL21
[0075] Take 1μl of the pET-Trx-CAD-Buforin II plasmid constructed in 2.1 above, dilute 10 times and directly transform E.coli expression strain BL21(DE3)plysS competent cells, spread LB+Amp plate, and invert overnight at 37℃.
[0076] 3.2 Screening of engineered strains expressing CAD-Buforin II
[0077] Pick 4 monoclonal colonies from the above-mentioned LB plate, culture in 2ml LB+Amp liquid medium with shaking at 37℃ overnight, and add 20μl of overnight culture to 2ml YTA+Amp liquid medium for transfer culture, shaking culture at 37℃ 3h, until the OD600 is between 0.5 and 0.7, then add IPTG to a final concentration of 0.3mM, culture with shaking at 30℃ to induce expression for 3-8h. Before induction, 0.5ml of bacterial solution was randomly taken from a sample and used as induction control d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com