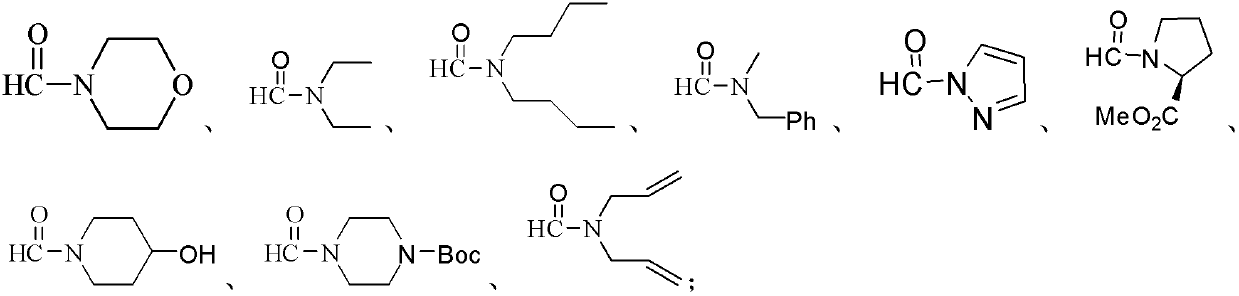
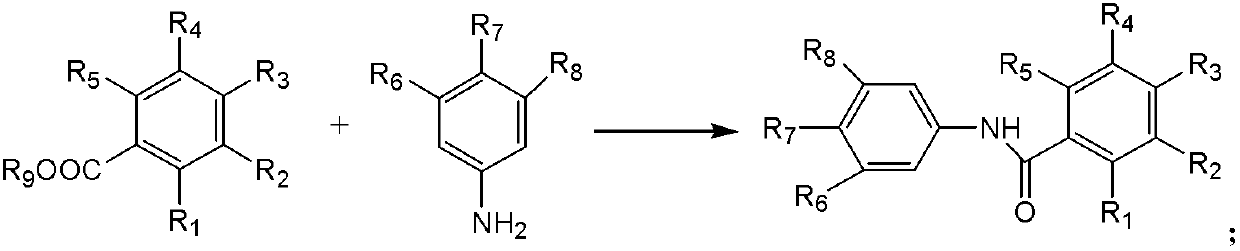
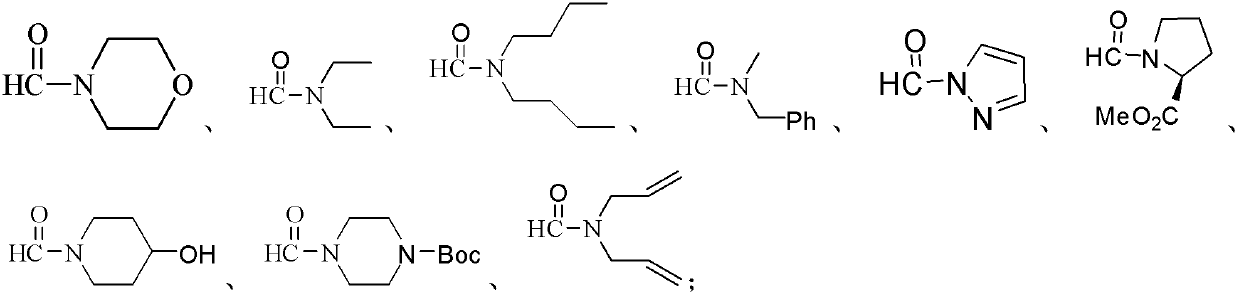
Preparation method of amide
A technology of amide and formamide, which is applied in the field of amide preparation, can solve the problems of restricting large-scale application, narrow use range of substrates, troublesome preparation of reactants, etc., and achieve the effects of improving utilization efficiency, wide use range and excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] The reaction flasks were sequentially charged with Bu 4 NI (20 mol%), compound 1a (2 mmol, 312 mg), TBHP (11.6 mmol), N,N-dimethylformamide (2.4 mL), acetonitrile 8 mL. Then the system was heated at 100°C in air for about 24 hours, quenched with saturated sodium sulfite, washed with water, extracted with ethyl acetate (40 mL×3), and the oxidized product 3a was obtained by simple column chromatography with a yield of 79%.
[0040] The product was analyzed and the results were as follows: 1 HNMR (300MHz, CDCl 3 ): δ7.88-7.64(m, 3H), 7.48-7.26(m, 4H), 3.16(s, 3H), 2.71(s, 3H). 13 CNMR (75MHz, CDCl 3): 170.8, 134.6, 133.3, 129.3, 128.9, 128.3, 126.9, 126.2, 125.1, 124.7, 123.8, 38.8, 34.8. MS: Anal.Calcd.For C 13 H 12 NO: 199, Found: 199 (M + ); IR(KBr, cm -1 ): v1621. The above data proves that the obtained compound is the target product.
Embodiment 2
[0042]
[0043] The reaction flasks were sequentially charged with Bu 4 NI (20 mol%), compound 1a (2 mmol, 312 mg), TBHP (2 mmol), N,N-dimethylformamide (2.4 mL), toluene 8 mL. Then the system was heated at 90°C in the air for about 24 hours, quenched with saturated sodium sulfite, washed with water, extracted with ethyl acetate (40 mL×3), and the oxidized product 3a was obtained by simple column chromatography with a yield of 85%.
[0044] The product was analyzed and the results were as follows: 1 HNMR (300MHz, CDCl 3 ): δ7.88-7.64(m, 3H), 7.48-7.26(m, 4H), 3.16(s, 3H), 2.71(s, 3H). 13 CNMR (75MHz, CDCl 3 ): 170.8, 134.6, 133.3, 129.3, 128.9, 128.3, 126.9, 126.2, 125.1, 124.7, 123.8, 38.8, 34.8. MS: Anal.Calcd.For C 13 H 12 NO: 199, Found: 199 (M + ); IR(KBr, cm -1 ): v1621. The above data proves that the obtained compound is the target product.
Embodiment 3
[0046]
[0047] The reaction flasks were sequentially charged with Bu 4 NI (20 mol%), compound 1a (2 mmol, 312 mg), TBHP (2 mmol), N,N-dimethylformamide (2.4 mL), acetonitrile 8 mL. Then the system was heated at 90°C in the air for about 24 hours, quenched with saturated sodium sulfite, washed with water, extracted with ethyl acetate (40 mL×3), and the oxidized product 3a was obtained by simple column chromatography with a yield of 59%.
[0048] The product was analyzed and the results were as follows: 1 HNMR (300MHz, CDCl 3 ): δ7.88-7.64(m, 3H), 7.48-7.26(m, 4H), 3.16(s, 3H), 2.71(s, 3H). 13 CNMR (75MHz, CDCl 3 ): 170.8, 134.6, 133.3, 129.3, 128.9, 128.3, 126.9, 126.2, 125.1, 124.7, 123.8, 38.8, 34.8. MS: Anal.Calcd.For C 13 H 12 NO: 199, Found: 199 (M + ); IR(KBr, cm -1 ): v1621. The above data proves that the obtained compound is the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com