Preparation method of conjugated linoleic acid glycerides
A technology of conjugated linoleic acid glycerides and conjugated linoleic acid, which is applied in the field of preparation of conjugated linoleic acid glycerides, can solve the problem of low production efficiency, reaction time of up to 20 hours or even 75 hours, and high cost Inappropriate and other problems, to achieve the effect of short reaction time, simple post-processing, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
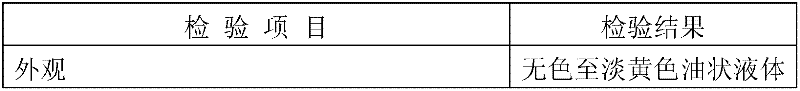
[0036] 1. Under the situation of blowing nitrogen gas, add dropwise 4.4 kg of 24% potassium ethoxide ethanol solution to 50 kg of methyl linoleate at 100°C; react for 3 hours, and finish the reaction with a methyl linoleate content of 1.1%;
[0037] ② After cooling down, add 50 liters of 60% methanol aqueous solution to wash the reactants in ①; let stand to separate liquid, and decompress and precipitate the oil layer;
[0038]③Set the molecular distillation heating temperature at 160°C; control the vacuum degree at 20Pa, and distill to obtain refined conjugated methyl linoleate.
[0039] ④ After mixing methyl conjugated linoleate and glyceryl acetate in a molar ratio of 3.1:1, raise the temperature to 125°C under nitrogen protection, and add the catalyst potassium methoxide solution dropwise. 3%, catalyze the ester exchange reaction, and react until the triglyceride content is qualified to start the high vacuum reaction to the end;
[0040] ⑤ Add 50 liters of 60% methanol aq...
Embodiment 2
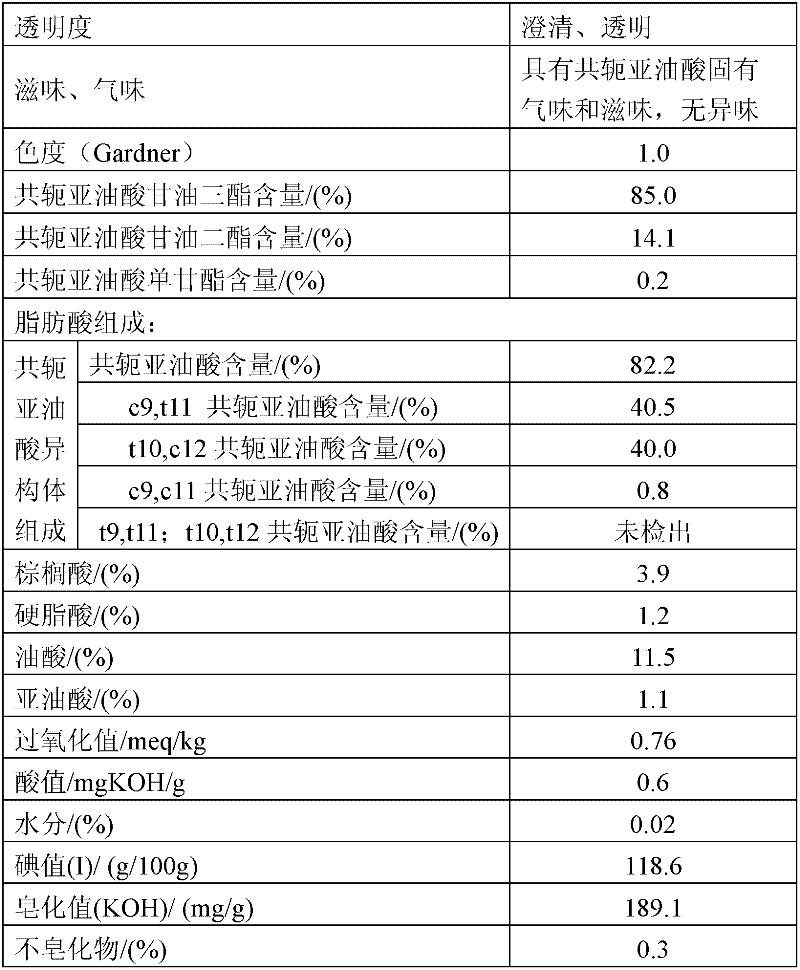
[0047] 1. Under the situation of blowing nitrogen gas, add dropwise 4.73 kg of 23.8% concentration of potassium ethoxide ethanol solution to 75 kg of ethyl linoleate at 110°C; react for 2 hours, and the content of methyl linoleate is 1.14% to end the reaction;
[0048] ② After cooling down, add 7.5 liters of 30% aqueous ethanol solution to wash the reactants in ①; let stand to separate liquid, and decompress and precipitate the oil layer;
[0049] ③Set the molecular distillation heating temperature at 175°C; control the vacuum degree at 24±2Pa, and distill to obtain refined conjugated ethyl linoleate;
[0050] ④ After mixing ethyl conjugated linoleate and glyceryl acetate in a molar ratio of 3.2:1, raise the temperature to 145°C under the protection of nitrogen, and add the catalyst potassium ethylate solution dropwise. The dosage of potassium ethylate is changed to glyceryl acetate 4% of the mass, catalyze the transesterification reaction, and react until the triglyceride con...
Embodiment 3
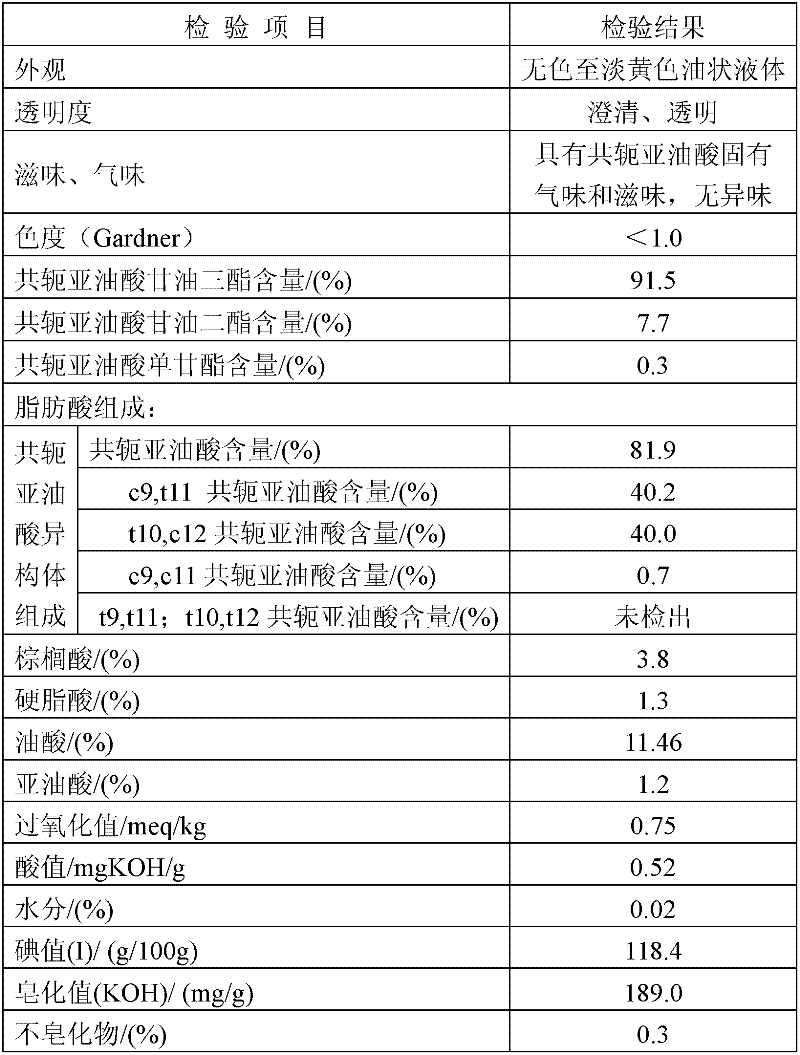
[0057] 1. In the case of nitrogen blowing, add dropwise 187.5 kg of 20.8% concentration of sodium methoxide methanol solution to 500 kg of ethyl linoleate at 140°C; react for 3.5 hours, and the methyl linoleate content is 0.95% to end the reaction;
[0058] ② After cooling down, add 450 liters of 70% aqueous ethanol solution to wash the reactants in ①; let stand to separate the liquid, and decompress and precipitate the oil layer;
[0059] ③Set the molecular distillation heating temperature at 165°C; control the vacuum degree at 20Pa, and distill to obtain refined conjugated ethyl linoleate.
[0060] ⑤ After mixing ethyl conjugated linoleate and glyceryl acetate in a molar ratio of 3.5:1, raise the temperature to 150°C under nitrogen protection, and add the catalyst sodium ethoxide solution dropwise. The amount of sodium ethoxide is the mass of glyceryl acetate 0.8%, catalyze the transesterification reaction, react until the triglyceride content is qualified, start the high va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com