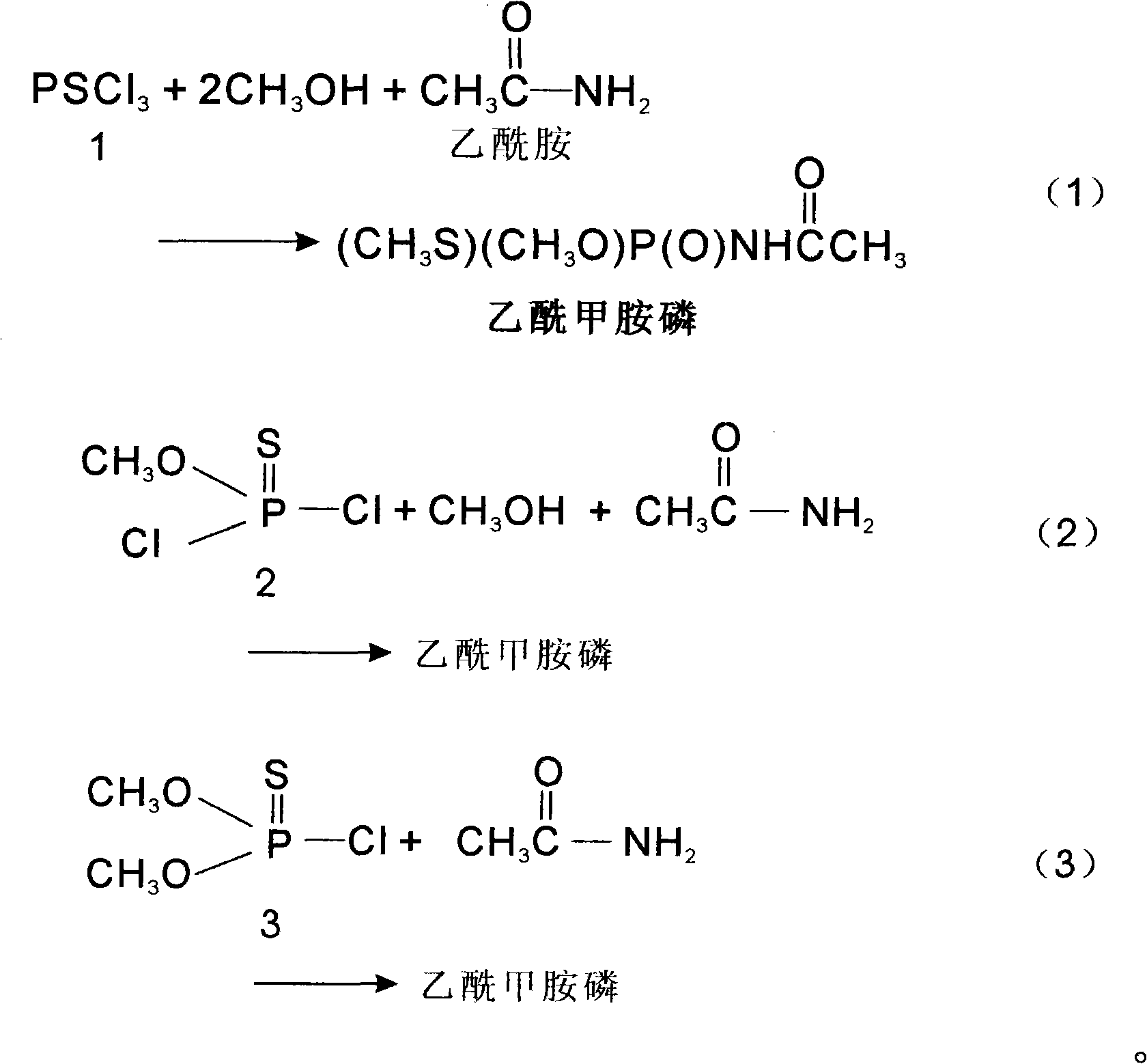
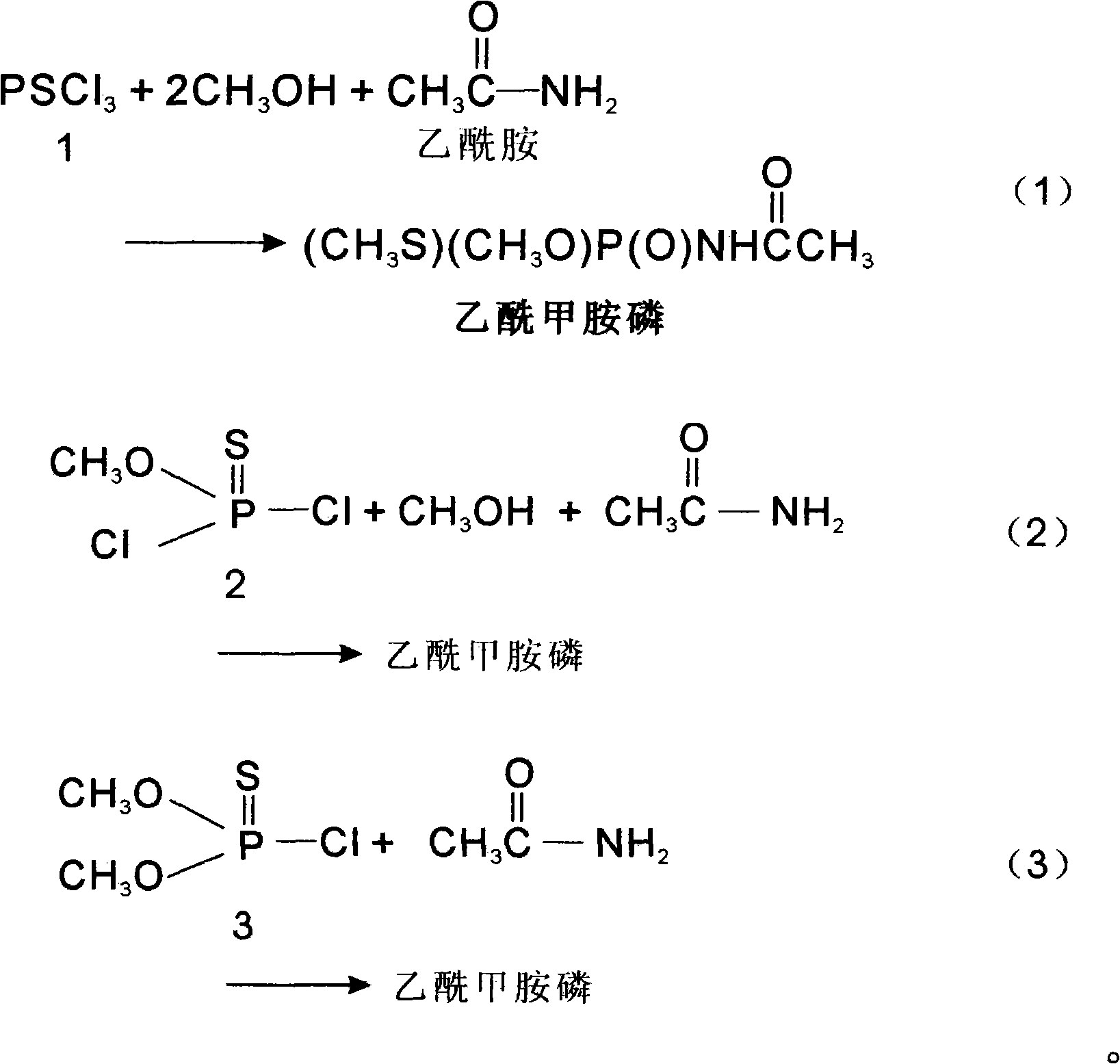
Method for synthesizing oxydemeton-methyl by taking phosphorus oxychloride as raw material through reaction in one step
A technology for acephate and acetamide is applied in the field of one-step reaction synthesis of acephate using phosphorus oxychloride as a raw material, and can solve the problems of large waste water discharge, high refrigeration energy consumption, high environmental control pressure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Molecular ratio: PSCl 3 Or O-methylphosphoryl dichloride or O, O-dimethylphosphoryl chloride:methanol:acetamide:sodium hydroxide (or X)=1:2~15:1:1~3
[0018] Put the calculated amount of acetamide, methanol and sodium hydroxide or X into the reaction bottle at one time, start stirring, control the temperature at 30-40°C, and drop the calculated amount of PSCl 3 , heat preservation reaction for 4 to 32 hours, cooling, standing, filtering, removing sodium chloride solid, distilling the filtered mother liquor, distilling off excess methanol solvent, cooling, standing, filtering, and drying to obtain acephate Drug solid, yield 90%, content 97%, filter the mother liquor for mechanical use.
[0019] In Example 1, X represents acid-binding agents such as potassium hydroxide, sodium acetate, sodium benzoate, sodium methoxide, N, N-dimethylformamide, ammonium acetate, or N, N-dimethylaniline.
Embodiment 2
[0021] Molecular ratio: PSCl 3 Or O-methylphosphoryl dichloride or O, O-dimethylphosphoryl chloride: methanol: acetamide: sodium hydroxide (or X) = 1:1~2:1:1~3
[0022] Weight ratio: acetamide: ether (or Y) = 1:9
[0023] Put the calculated amount of acetamide and ether or Y and methanol and sodium hydroxide or X into the reaction bottle at one time, start stirring, control the temperature at about 30°C, and drop the calculated amount of PSCl 3 , heat preservation reaction for 28 hours, lower the temperature, stand still, filter, and dry to obtain a solid mixture of acephate and sodium chloride, filter the mother liquor for mechanical use, dissolve acephate in the mixture solid with methanol, and remove the solid sodium chloride by filtration , and then distill the methanol solvent of the filtered mother liquor, lower the temperature, stand still, filter, and dry to obtain the original drug of acephate, with a content of 98% and a yield of 95%.
[0024] X represents the same...
Embodiment 3
[0026] Molecular ratio: PSCl 3 Or O-methylphosphoryl dichloride or O, O-dimethylphosphoryl chloride:methanol:acetamide=1:1~2:1
[0027] Weight ratio: acetamide: propyl ether (or Y) = 1:9
[0028] Put the calculated amount of acetamide and propyl ether or Y into the reaction flask at one time, start stirring, control the temperature at 30-40°C, and drop the calculated amount of PSCl 3 When the content of acetamide in the reaction system is tested by liquid chromatography normalization method 1≤%, the temperature is raised to control the temperature to reflux, the calculated amount of methanol or sodium methylate is added dropwise, and the methanol content in the reaction system is tested by gas chromatography normalization method≤1% , lower the temperature, stand still, filter, and dry to obtain the original drug of acephate, with a content of 95% and a yield of 90%, and filter the mother liquor for mechanical use.
[0029] The meaning of Y is the same as that of Y in Example...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com