Biological enzyme resolution method for preparing optically pure (S)-5-(4-fluorophenyl)-5-hydroxypentanoate
A technology of methyl hydroxyvalerate and methyl acetoxyvalerate, which is applied in the field of biocatalytic preparation of chiral drug intermediates, can solve problems such as effective utilization and inability to recycle splits, achieve high optical purity, and improve resolution The effect of high efficiency and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
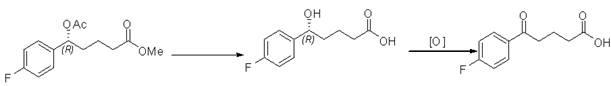
[0025] Example 1 ( RS )-5-(4-fluorophenyl)-5-hydroxypentanoic acid methyl ester preparation
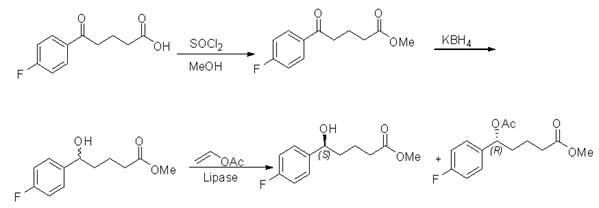
[0026] Add 42g (0.2mol) of p-fluorobenzoylbutyric acid into 400ml of anhydrous methanol, cool in an ice bath to 0°C, slowly add 29ml (0.4mol) of thionyl chloride dropwise, keep the temperature below 10°C during the dropwise addition, and drop After addition, reflux at 50°C for 2-3 hours, distill methanol and thionyl chloride under reduced pressure, dissolve the residue with 150ml ethyl acetate, wash with 150ml saturated sodium bicarbonate and 150ml saturated brine, and dry over anhydrous sodium sulfate , and concentrated to dryness to obtain 40 g of methyl p-fluorobenzoyl butyrate (light yellow solid), with a yield of 89%.
[0027] Add 22.4g (0.1mol) of methyl p-fluorobenzoylbutyrate into 300ml of anhydrous methanol, cool in an ice bath to 0°C, add 3.8g (0.1mol) of sodium borohydride in batches, react at room temperature for 20min, and finish the reaction with 1mol / L dilute hydroch...
Embodiment 2
[0028] Example 2 ( RS )-5-(4-fluorophenyl)-5-hydroxypentanoic acid methyl ester resolution
[0029] Add ( RS )-5-(4-fluorophenyl)-5-hydroxypentanoic acid methyl ester 11.3g (0.05mol), Lipozyme TL IM 5.65g, vinyl acetate 8.6g (0.1mol), methyl tert-butyl ether 60ml, React at 40 °C on a shaking table (160 r / min) for 72 h. After the reaction was completed, the enzyme was removed by filtration, the filtrate was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (n-hexane:ethyl acetate=1:1, V / V) to obtain ( S )-5-(4-fluorophenyl)-5-hydroxypentanoic acid methyl ester 5.1g, yield 45%, ee 99%, ( R )-5-(4-fluorophenyl)-5-acetoxyvaleric acid methyl ester 6.16g, yield 46%, ee 99%.
Embodiment 3
[0030] Example 3 ( R )-5-(p-Fluorophenyl)-5-acetoxyvaleric acid methyl ester racemization
[0031] Add 6.16g ( R )-5-(4-fluorophenyl)-5-acetoxypentanoic acid methyl ester (0.023mol), 20ml tetrahydrofuran and 2mol / L sodium hydroxide solution 46ml (0.092mol), react at room temperature for 1h, and use Adjust the pH to 2-3 with 2mol / L hydrochloric acid, extract with ethyl acetate (2×100ml), wash with 100ml saturated brine, dry over anhydrous sodium sulfate, and concentrate to obtain 4.63g ( R )-5-(p-fluorophenyl)-5-hydroxypentanoic acid, yield 95%.
[0032] Add ( R )-5-(4-fluorophenyl)-5-hydroxypentanoic acid 4.63g (0.022mol), 40ml dimethylformamide and 16.8g pyridinium dichromate (0.022mol), react for 3h, add 100ml ethyl acetate ester, the organic layer was washed with 100ml of saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 3.7g of p-fluorobenzoyl butyric acid, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com