Detection method of angiotensin converting enzyme (ACE) inhibitory activity of antihypertensive peptide
An angiotensin and inhibitory activity technology, applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as inability to detect ACE inhibitory activity, and achieve the effect of saving detection time and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
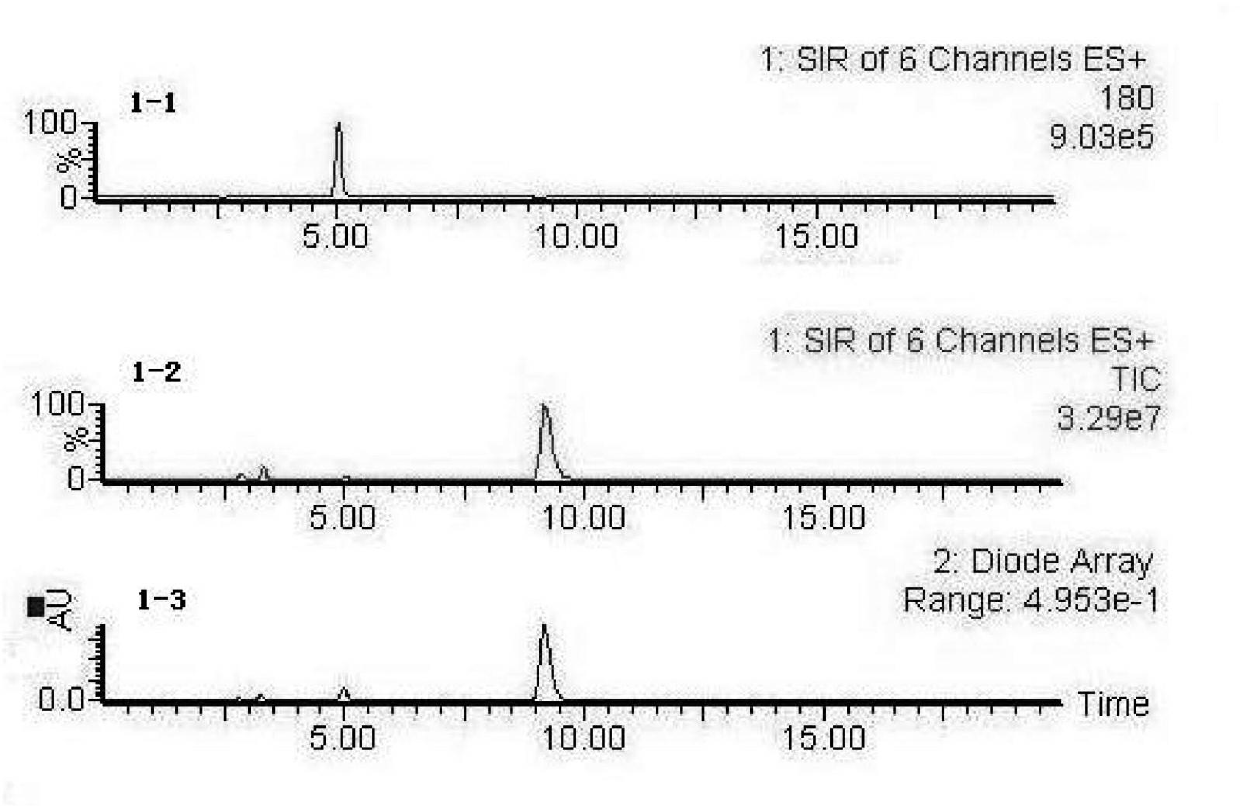
[0027] 1. Dissolve a certain amount of fish scale antihypertensive peptide in pH 8.3, 0.1mol / L boric acid buffer, prepare a sample solution with a concentration of 1mg / ml, absorb 25μL of antihypertensive peptide, and add 75μL of 60mU·ml -1 The ACE was incubated at 37°C for 5min, and then 112μL of 5.0mmol / L HHL was added. In the blank control group, the volume of the antihypertensive peptide was replaced by the same volume of boric acid buffer solution, incubated in a constant temperature water bath at 37°C for 60min, and then 25μL of 0.1 %TFA terminated the reaction.
[0028] 2. LC-MS detection and analysis of reaction products, high performance liquid chromatography conditions: Ug120-C18 reversed-phase chromatographic column, mobile phase is acetonitrile: ultrapure water = 20:80 (containing 0.1% TFA, volume ratio), flow rate 1.0mL·min -1 , The detection wavelength is 228nm, and the injection volume is 10μL. The mass spectrometry conditions are: electrospray ionization posit...
example 2
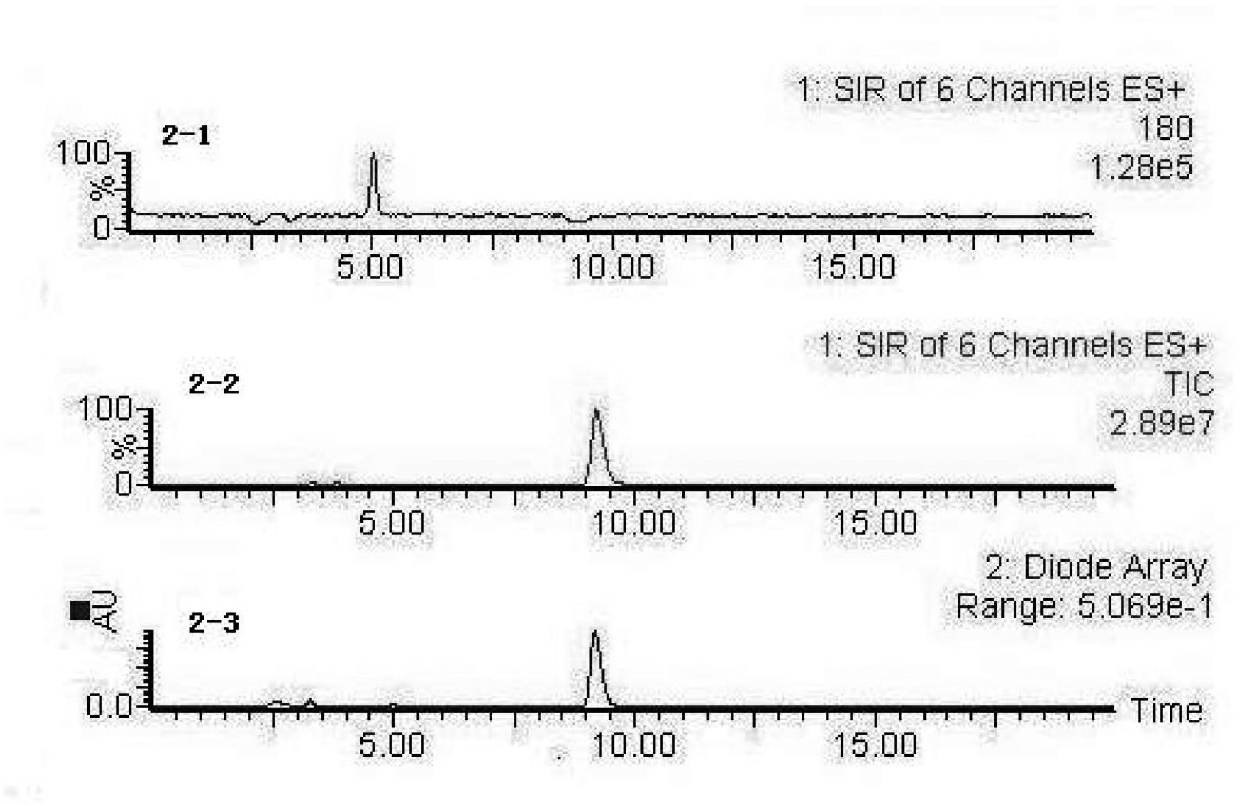
[0031] 1. Dissolve a certain amount of peanut antihypertensive peptide in pH 8.3, 0.1mol / L boric acid buffer, prepare a sample solution with a concentration of 1mg / ml, absorb 25μL of antihypertensive peptide, and add 75μL of 60mU·ml -1 The ACE was incubated at 37°C for 5min, and then 112μL of 5.0mmol / L HHL was added. In the blank control group, the volume of the antihypertensive peptide was replaced by the same volume of boric acid buffer solution, incubated in a constant temperature water bath at 37°C for 60min, and then 25μL of 0.1 %TFA terminated the reaction.
[0032]2. LC-MS detection and analysis of reaction products, high performance liquid chromatography conditions: Ug120-C18 reversed-phase chromatographic column, mobile phase is acetonitrile: ultrapure water = 20:80 (containing 0.1% TFA) (volume ratio), flow rate 1.0mL·min -1 , The detection wavelength is 228nm, and the injection volume is 10μL. The mass spectrometry conditions are: electrospray ionization positive ...
example 3
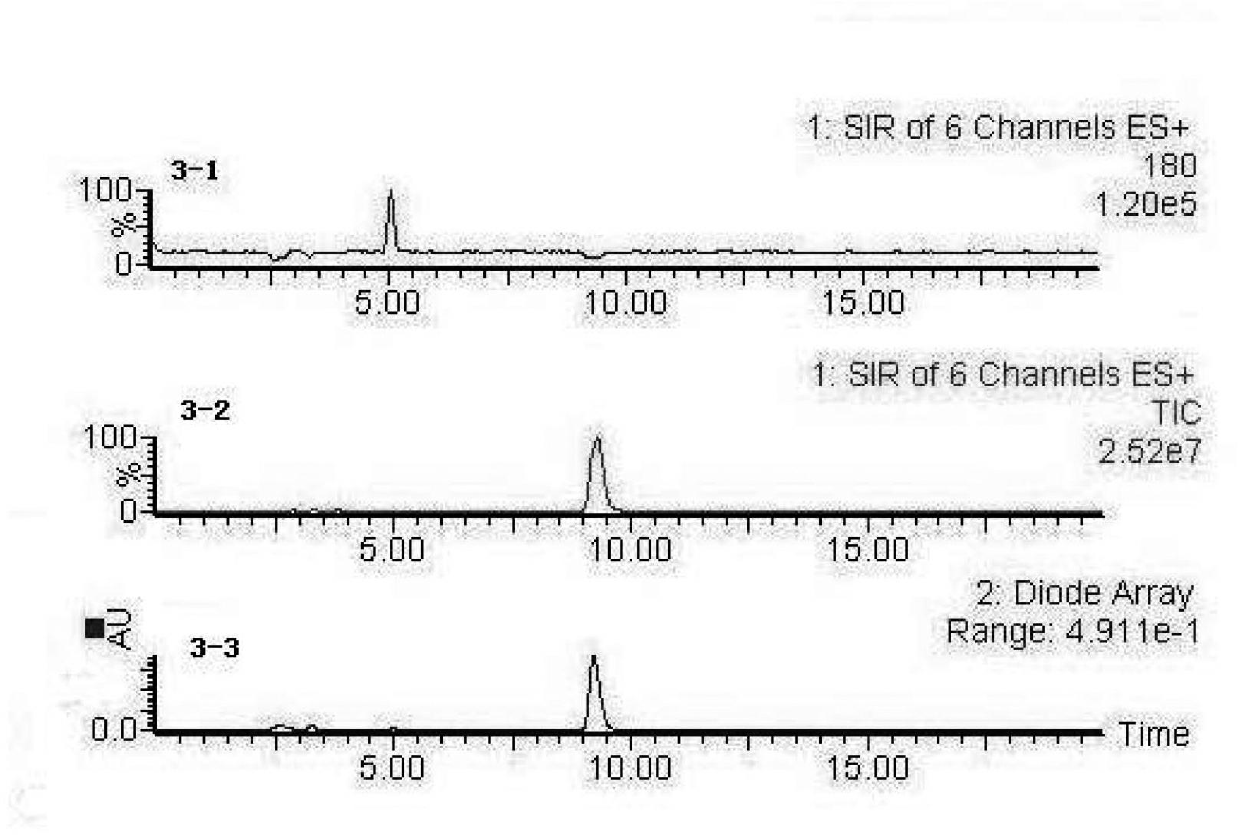
[0035] 1、 Dissolve a certain amount of glycine-phenylalanine (Gly-Phe) in pH 8.3, 0.1mol / L boric acid buffer, prepare a sample solution with a concentration of 1mg / ml, absorb 25μL of antihypertensive peptide, add 75μL60mU·ml -1 The ACE was incubated at 37°C for 5min, and then 225μL of 5.0mmol / L HHL was added. In the blank control group, the same volume of boric acid buffer was used to replace the volume of the antihypertensive peptide, incubated in a constant temperature water bath at 37°C for 60min, and then 25μL of 0.1 %TFA terminated the reaction.
[0036] 2、 LC-MS detection and analysis of reaction products, high performance liquid chromatography conditions: Ug120-C18 reversed-phase chromatographic column, mobile phase is acetonitrile: ultrapure water = 20:80 (containing 0.1% TFA) (volume ratio), flow rate 1.0mL min -1 , The detection wavelength is 228nm, and the injection volume is 10μL. The mass spectrometry conditions are: electrospray ionization positive ion mode,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com