Lanthanum hexaboride nanowire and method for preparing same
A technology of lanthanum hexaboride and nanowires, which is applied in the field of preparing lanthanum hexaboride nanowires, can solve the problems of poor shape, low output, and increased production costs, and achieve industrial production, high output, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Put the copper substrate into a small beaker, add a little ethanol, and ultrasonically clean it for 30 minutes; then add acetone to clean it; and finally dry it naturally in the air for use.
[0027] (2) Weigh about 0.1g of LaCl 3 *7H 2 O, put into the bottom of the small quartz test tube, and place the small quartz test tube in the middle of the quartz tube of the electric tube furnace.
[0028] (3) Put the aforementioned copper substrate on the quartz plate, and put the quartz plate into the downflow direction of the quartz tube precursor of the electric tube furnace; seal the quartz tube, fill it with protective and reducing gas, and then vacuumize the quartz tube.
[0029] (4) Heat the quartz tube to 900° C. at a heating rate of about 15° C. / min under vacuum conditions, and feed a boron source at a flow rate of 10 sccm; keep for 10 minutes.
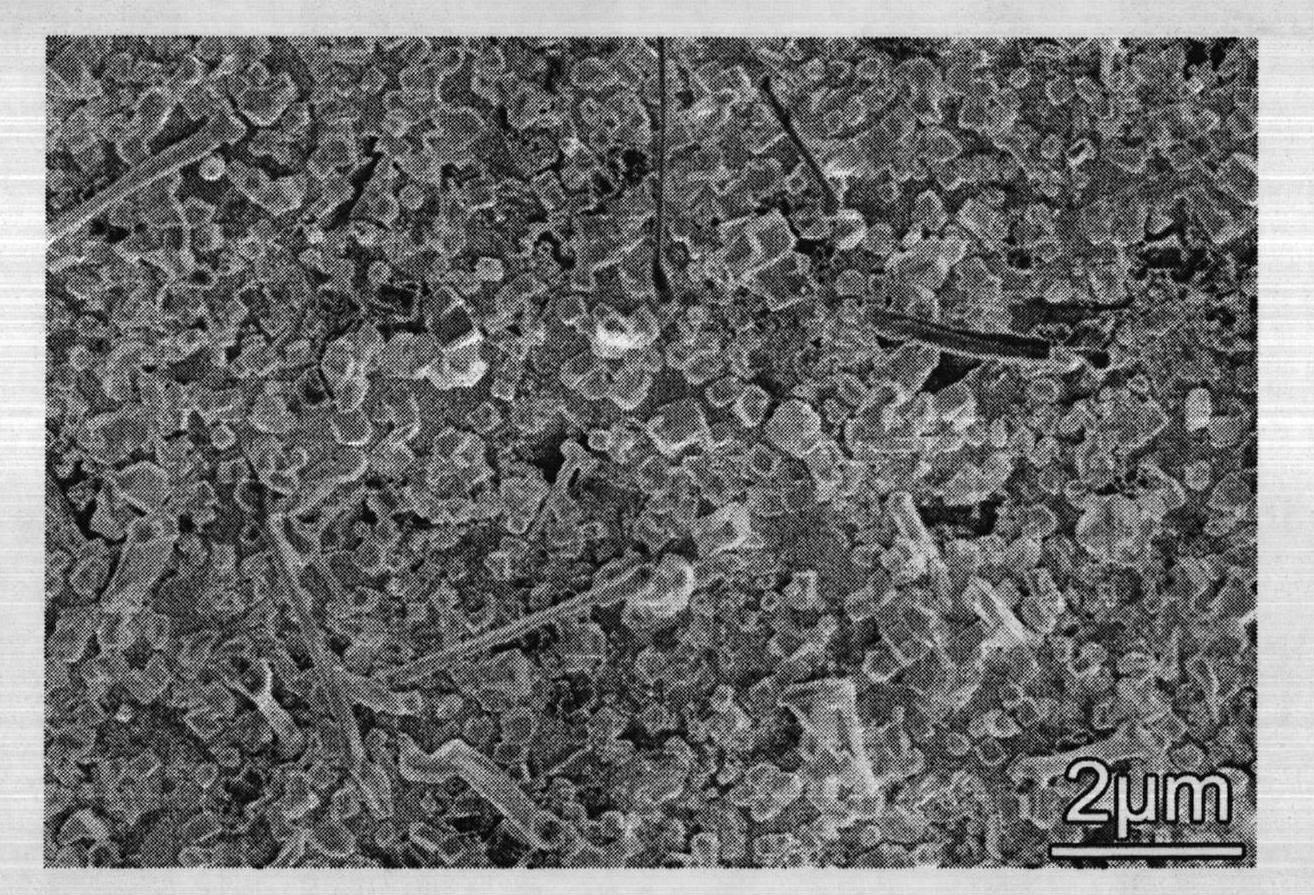
[0030] (5) Finally, the temperature is lowered in a vacuum atmosphere until it is cooled to room temperature; the coppe...
Embodiment 2
[0033] (1) Put the copper substrate into a small beaker, add a little ethanol, and ultrasonically clean it for 30 minutes; then add acetone to clean it; and finally dry it naturally in the air for use.
[0034] (2) Weigh about 0.2g of LaCl 3 *7H 2 O, put into the bottom of the small quartz test tube, and place the small quartz test tube in the middle of the quartz tube of the electric tube furnace.
[0035] (3) Put the aforementioned copper substrate on the quartz plate, and put the quartz plate into the downflow direction of the quartz tube precursor of the electric tube furnace; seal the quartz tube, fill it with protective and reducing gas, and then vacuumize the quartz tube.
[0036] (4) Heat the quartz tube to 960° C. at a heating rate of about 15° C. / min under vacuum conditions, and feed a boron source at a flow rate of 30 sccm; keep for 20 minutes.
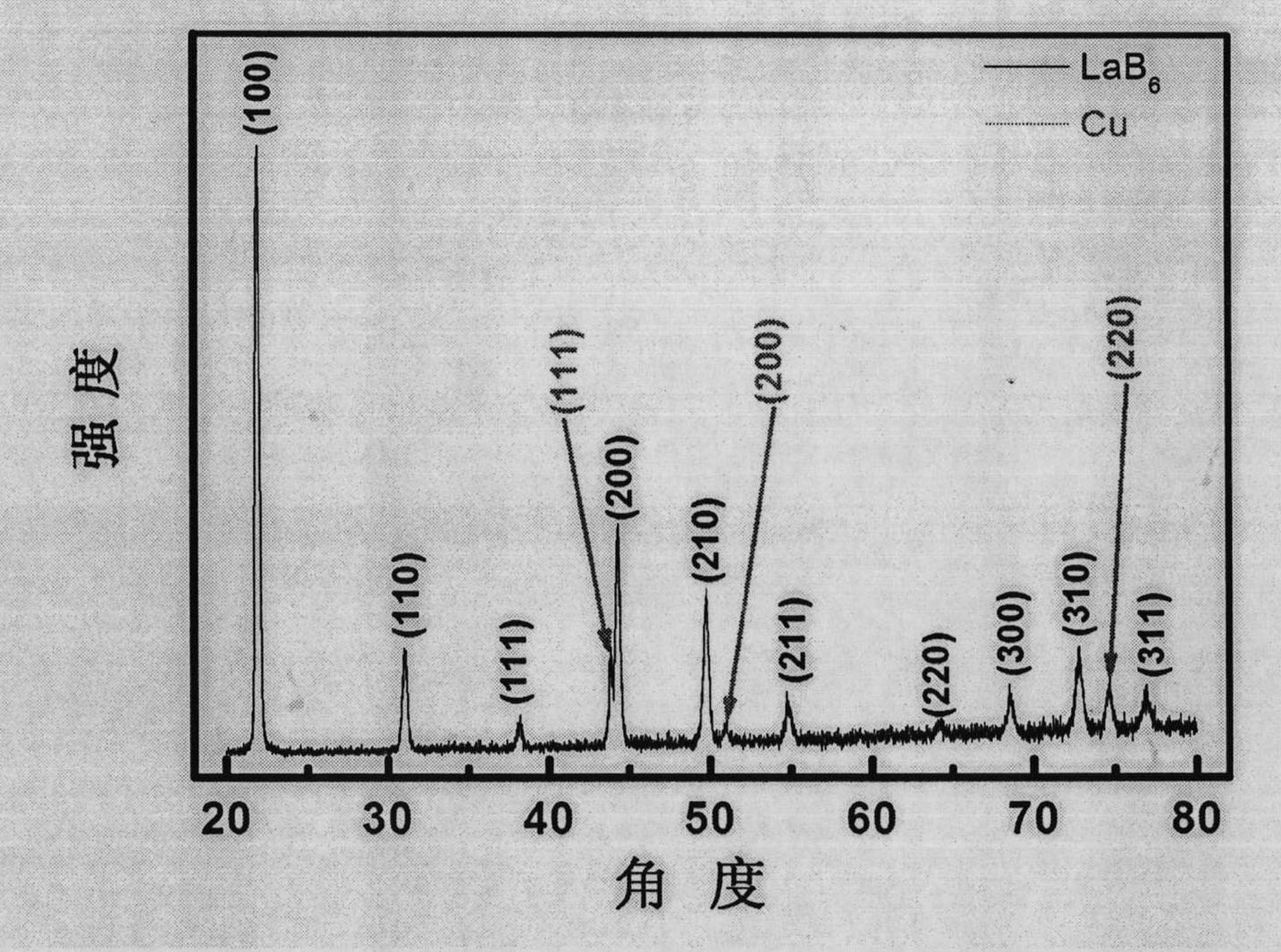
[0037] figure 2 is the X-ray diffraction (XRD) spectrum of the prepared sample. Diffraction peaks can be divided int...
Embodiment 3
[0043] (1) Put the copper substrate into a small beaker, add a little ethanol, and ultrasonically clean it for 30 minutes; then add acetone to clean it; and finally dry it naturally in the air for use.
[0044] (2) Weigh about 0.3g of LaCl 3 *7H 2 O, put into the bottom of the small quartz test tube, and place the small quartz test tube in the middle of the quartz tube of the electric tube furnace.
[0045] (3) Put the aforementioned copper substrate on the quartz plate, and put the quartz plate into the downflow direction of the quartz tube precursor of the electric tube furnace; seal the quartz tube, fill it with protective and reducing gas, and then vacuumize the quartz tube.
[0046] (4) Heat the quartz tube to 1020° C. at a heating rate of about 15° C. / min under vacuum conditions, and feed a boron source at a flow rate of 50 sccm; keep for 30 minutes.
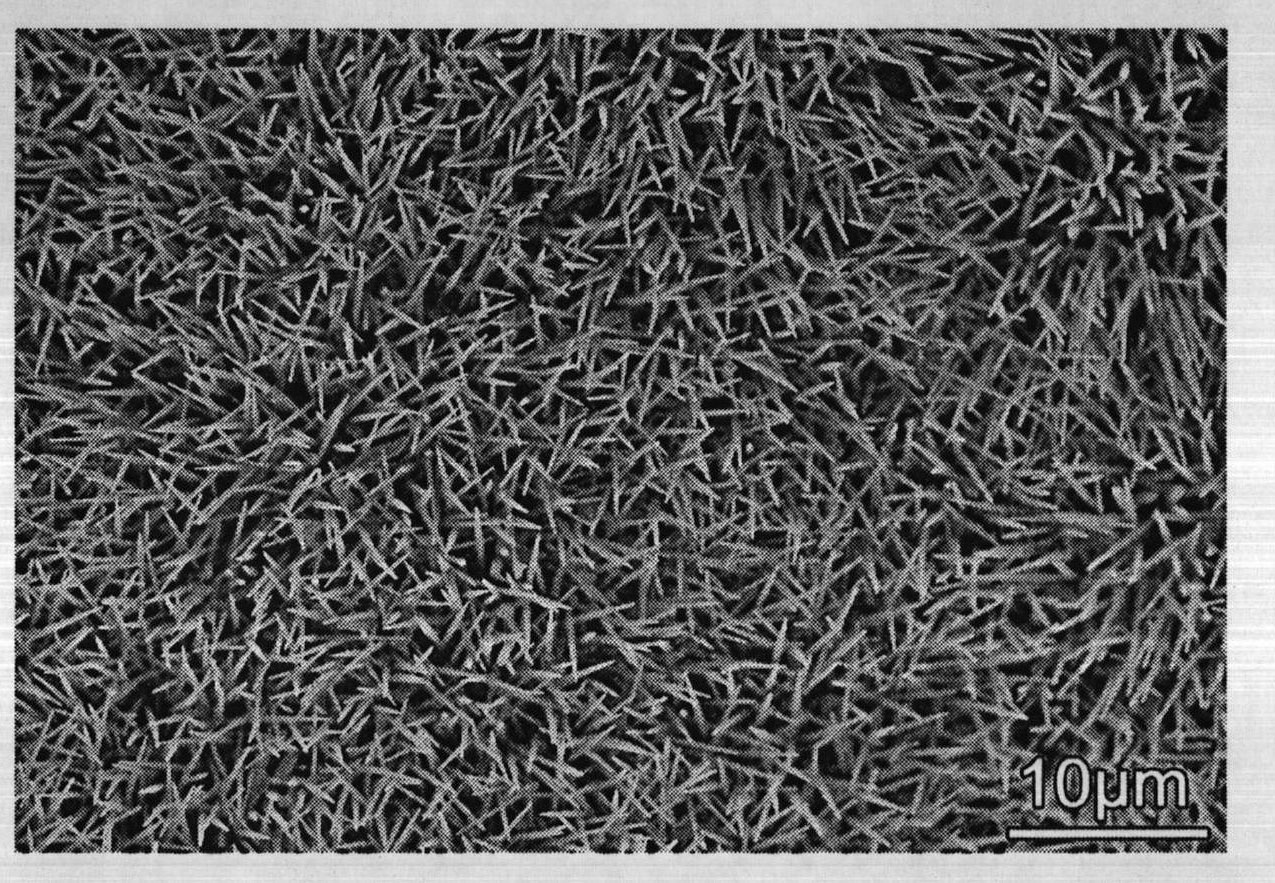
[0047] (5) Finally, the temperature is lowered in a vacuum atmosphere until it is cooled to room temperature; the copp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com