N-hydroxy ester preparation method
A hydroxyester and hydroxyl technology, applied in the field of preparing N-hydroxyesters, can solve the problems of cumbersome catalytic system, cumbersome steps, high price, etc., and achieve the effects of wide range of use, mild reaction conditions, and improved utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
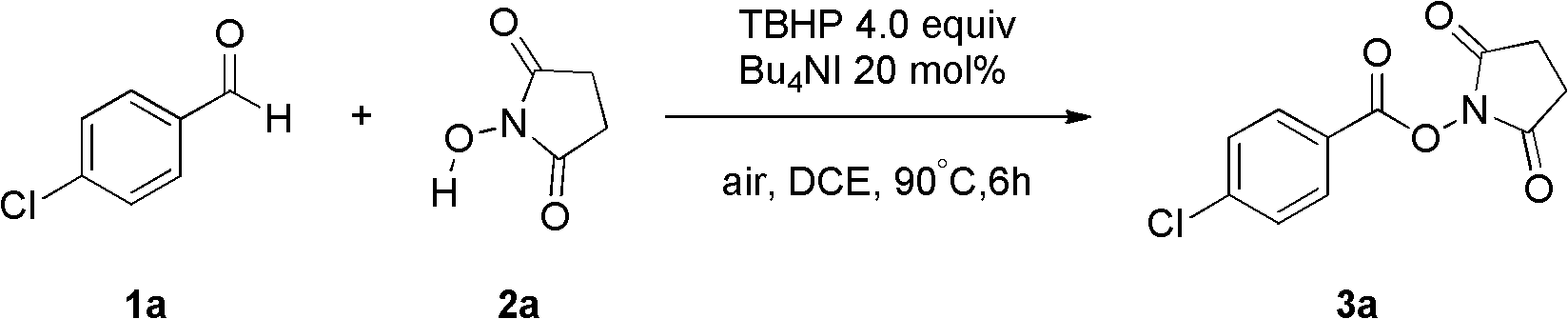
[0032] Fill the reaction bottle with Bu 4 NI (20 mol%), compound 1a (2 mmol, 280 mg), TBHP (4 equiv., 1.2 mL), N-hydroxysuccinimide (2.5 equiv., 576 mg), 1,2-dichloroethane 15 mL. Then the system was heated at 90°C in the air for about 6 hours, quenched with saturated sodium sulfite, extracted with ethyl acetate (40mL×3), and the oxidation product 3a was obtained by simple column chromatography with a yield of 98%. .
[0033] The product is analyzed and the results are as follows: 1 H NMR (400MHz, CDCl 3 )δ=8.08(d, J=8.6Hz, 2H), 7.50(d, J=8.6Hz, 2H), 2.92(s, 4H); 13 C NMR (75MHz, CDCl 3 )δ=169.2, 161.1, 141.6, 131.8, 129.3, 123.5, 25.6; MS (ESI) m / z calcd for C 11 h 8 35 ClNNaO 4 (M+Na)276, found 276, C 11 h 8 37 ClNNaO 4 (M+Na) 278, found 278; IR (KBr, cm -1 ): v 1731, 1596. The above data prove that the obtained compound is the target product.
Embodiment 2
[0035]
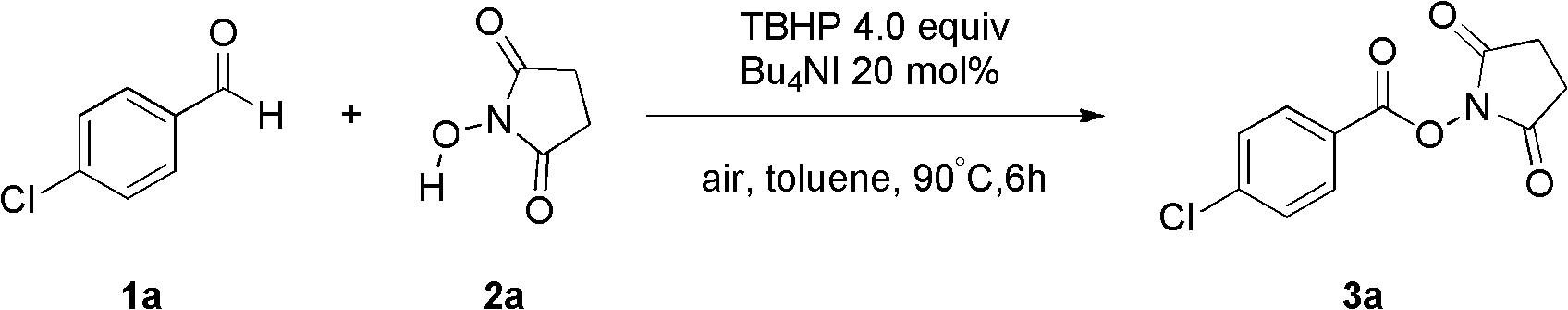
[0036] Fill the reaction bottle with Bu 4 NI (20 mol%), compound 1a (2 mmol, 280 mg), TBHP (4 equiv., 1.2 mL), N-hydroxysuccinimide (2.5 equiv., 576 mg), toluene 15 mL. Then the system was heated at 90°C in air for about 6 hours, quenched with saturated sodium sulfite, extracted with ethyl acetate (40mL×3), and the oxidation product 3a was obtained by simple column chromatography with a yield of 66%. .
[0037] The product is analyzed and the results are as follows: 1 H NMR (400MHz, CDCl 3 )δ=8.08(d, J=8.δHz, 2H), 7.50(d, J=8.6Hz, 2H), 2.92(s, 4H); 13 C NMR (75MHz, CDCl 3)δ=169.2, 161.1, 141.6, 131.8, 129.3, 123.5, 25.6; MS (ESI) m / z calcd for C 11 h 8 35 ClNNaO 4 (M+Na)276, found 276, C 11 h 8 37 ClNNaO 4 (M+Na) 278, found 278; IR (KBr, cm -1 ): v 1731, 1596. The above data prove that the obtained compound is the target product.
Embodiment 3
[0039]
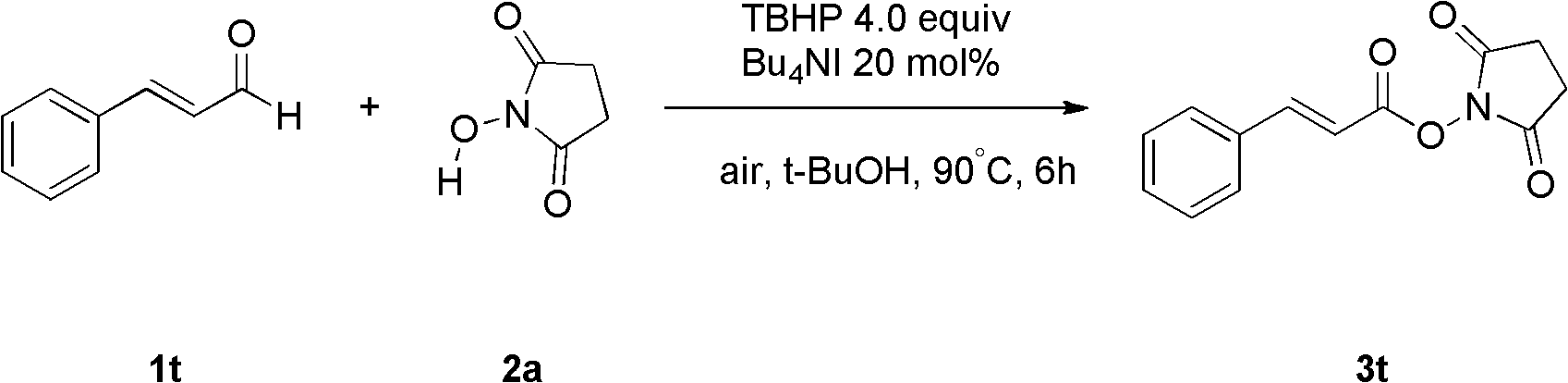
[0040] Fill the reaction bottle with Bu 4 NI (20 mol%), compound 1t (2 mmol, 264 mg), TBHP (4 equiv., 1.2 mL), N-hydroxysuccinimide (2.5 equiv., 576 mg), tert-butanol 15 mL. Then the system was heated in the air at 90°C for about 6 hours, quenched with saturated sodium sulfite, extracted with ethyl acetate (40mL×3), and the oxidation product 3t was obtained by simple column chromatography with a yield of 79%. .
[0041] The product is analyzed and the results are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.92(d, J=15.9Hz, 1H), 7.57(d, J=7.4Hz, 2H), 7.48-7.41(m, 3H), 6.59(d, J=15.9Hz, 1H), 2.88( s,4H); 13 C NMR (75MHz, d 6 -DMSO) δ = 170.4, 162.4, 149.9, 133.4, 131.7, 129.2, 129.1, 111.8, 25.5; MS (ESI) m / zcalcd for C 13 h 11 NNaO 4 (M+Na) 268, found 268; IR (KBr, cm -1 ): v 1758, 1627. The above data prove that the obtained compound is the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com