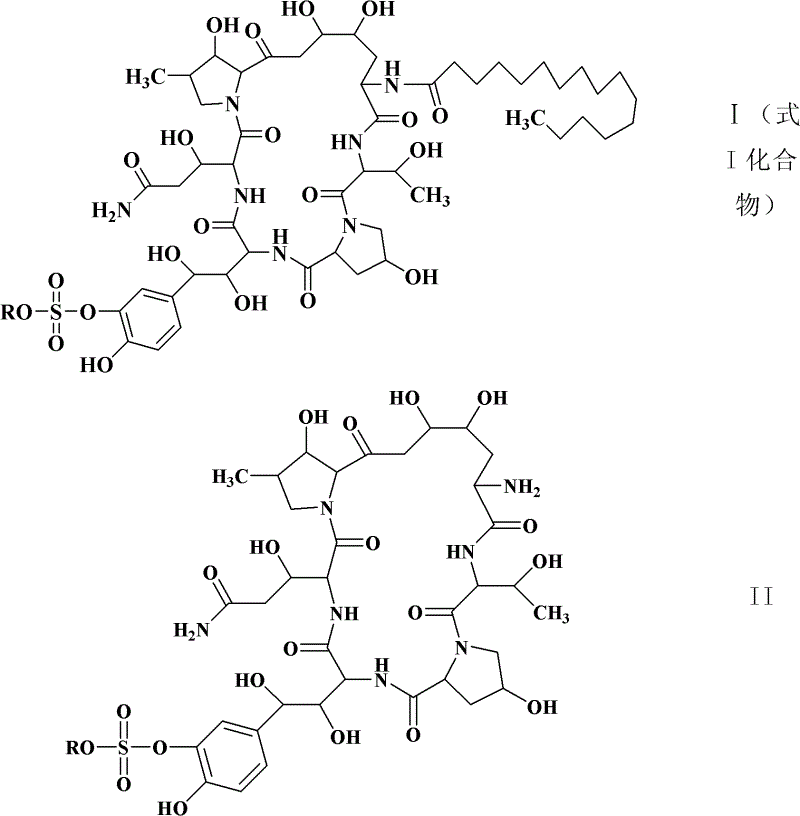
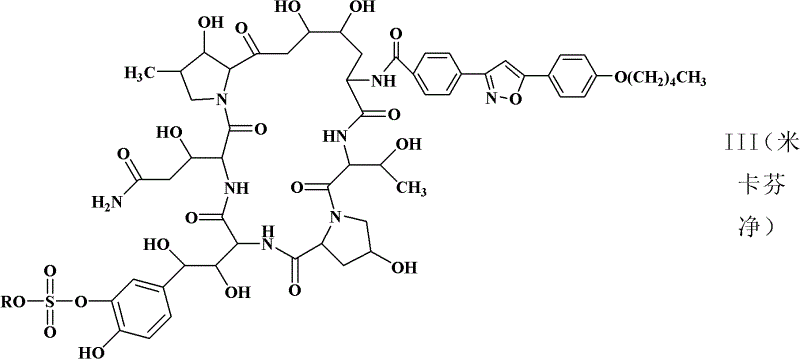
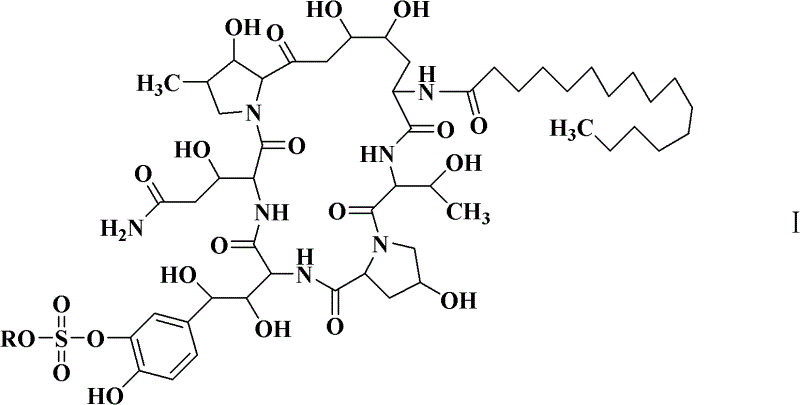
Preparation method of cyclic lipopeptide compound
A compound and amino acid technology, applied in the field of biosynthetic cycloaliphatic peptide compounds, can solve the problems of unfavorable dissolved oxygen control, subsequent filtration operation, and high medium viscosity, and achieve easy dissolved oxygen control, reduce production costs, and reduce environmental damage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Fermentation Strain Preparation Coleophoma empetri F-11899 (FERM BP2635)
[0059] The seed liquid of Coleophoma empetri F-11899 (FERMBP2635) was prepared with reference to the cultivation method of seeds in the document Scale-up fermentation of echinocandin type antibiotic FR901379 (Journal of Bioscience and Bioengineering, VOL 109 No. 2, 138-144, 2010), for later The compound of formula I is used for fermentation.
[0060] Potato dextrose agar medium (PDA) was used as the slant medium, and its composition was: 30% potato, 2% glucose, and 1.5% agar.
[0061] Seed medium composition: sucrose 1%, cottonseed meal 2%, dry yeast 1%, peptone 1%, KH 2 PO 4 0.2%, CaCO 3 0.2%, defoamer 0.05%.
[0062] Strain Coleophoma empetri F-11899 (FERM BP2635) matures after being cultivated on a slant at 25°C for 6-10 days, picks mature mycelia or spores and inserts them into the seed medium, and then cultivates them on a shaker at 25°C with a rotation speed of 280RPM for 2 -4 days. ...
Embodiment 2
[0064] Fermentation strain preparation (mutagenic strain CGMCC 4129)
[0065] With reference to the cultivation method of seeds in the document Scale-up fermentation of echinocandin type antibiotic FR901379 (Journal of Bioscience and Bioengineering, VOL 109 No.2, 138-144, 2010), the seed liquid of the mutagenized bacterial strain CGMCC 4129 was prepared to prepare the following formula I compound For fermentation.
[0066] Potato dextrose agar medium (PDA) was used as the slant medium, and its composition was: 30% potato, 2% glucose, and 1.5% agar.
[0067] Seed medium composition: sucrose 1%, cottonseed meal 2%, dry yeast 1%, peptone 1%, KH 2 PO 4 0.2%, CaCO 3 0.2%, defoamer 0.05%.
[0068] Strain CGMCC 4129 matures after being cultivated on a slant at 25°C for 6-10 days, picks mature mycelia or spores and inserts them into the seed medium, and then cultivates them on a shaker at 280RPM for 2-4 days at 25°C.
Embodiment 3
[0070] Preparation of compounds of formula I
[0071] In a 250ml shake flask, add 50ml containing mannitol concentration 5%, yeast extract concentration 0.5%, L-proline concentration 1%, cottonseed cake powder concentration 1%, ammonium sulfate concentration 0.1%, magnesium sulfate concentration 0.06%, Trace element solution concentration 0.1%, the culture medium of MES concentration 2%, adjust pH to be 5.5 ± 0.5, 121 ℃ of sterilization 30min, the seed 1ml (2%) that obtains in embodiment 1 is inoculated in this nutrient solution, 25 ℃ After cultivating at 280r / m for 240 hours, the cultivation was completed, and sampling analysis showed that the viscosity of the final culture solution was 2200cp, and the content of the compound of formula I was 0.5g / L.
[0072] Trace elements: FeSO 4 ·7H 2 O 10g / L, MnSO 4 ·H 2 O 10g / L, ZnSO 4 ·7H 2 O 2g / L, CaCl 2 0.7g / L,H 3 BO 3 0.56g / L, CuCl 22H 2 O 0.25g / L, (NH 4 ) 6 Mo 7 o 24 ·7H 2 O0.19g / L, concentrated hydrochloric acid 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com