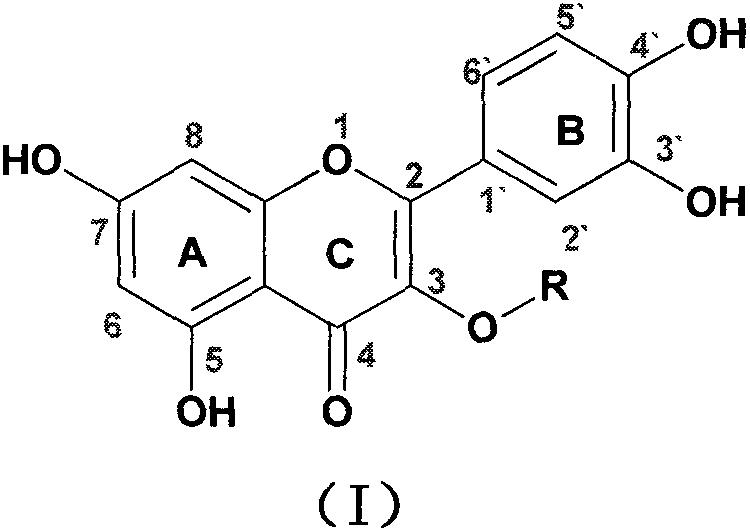
Quercetin-3-O-acyl ester and preparation method thereof
A technology of acyl ester and quercetin, applied in the field of chemical chemistry, can solve the problems of no anti-tumor activity, poor stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] 2 Preparation of compounds
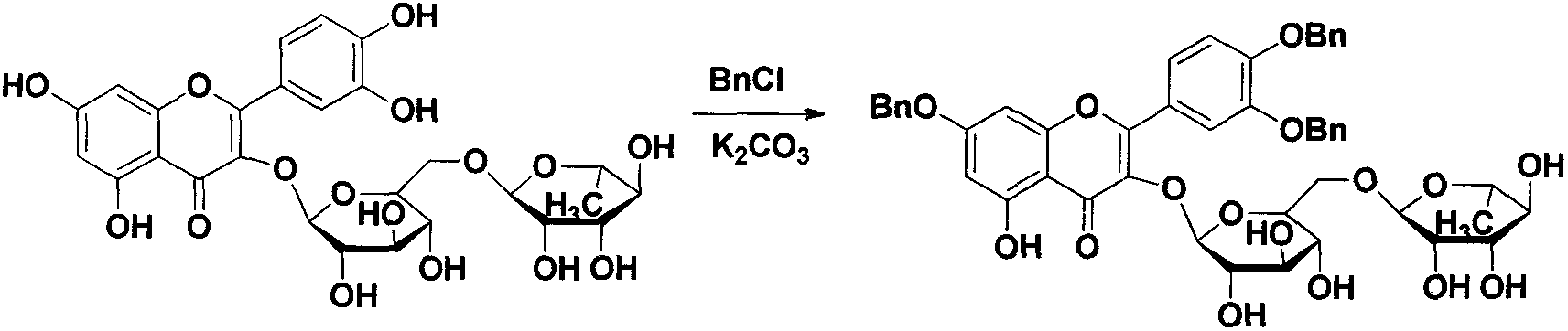
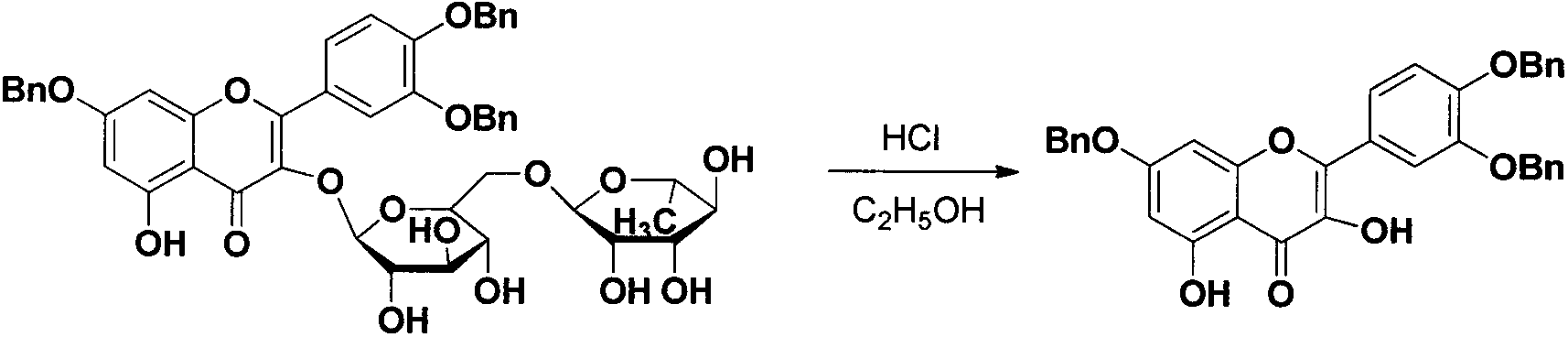
[0032] 2.17 Synthesis of 3`,4`-O-tribenzylquercetin
[0033] Take 2.44g (4.0mmol) of rutin and dissolve it in 20ml of DMF, add 1.93g (14.0mmol) of anhydrous potassium carbonate and stir, under ice bath conditions, slowly add 1.6ml (14.0mmol) of freshly distilled benzyl chloride dropwise, and slowly heat up After reaching room temperature, the reaction was stirred overnight (about 12 h), and the reaction was complete as detected by TLC. Add glacial acetic acid to adjust the pH of the reaction solution to 6, then add 200ml of distilled water to it, a large amount of precipitation precipitates out, after stirring for 1 hour, solids gather at the bottom of the bottle, and the supernatant is discarded. Add 60ml of 95% ethanol to the precipitate, heat in a water bath until the solid is completely dissolved, add 10ml of concentrated hydrochloric acid at one time, and heat to reflux. After 15 minutes, a yellow precipitate begins to form, and the re...
Embodiment 1
[0035] Embodiment 1: the preparation of quercetin-3-O-acetate (3a)
[0036] Synthesis of 7,3`,4`-O-tribenzylquercetin-3-O-acetate
[0037] Weigh 1.144g (2mmol) tribenzyl quercetin, dissolve it in 40ml of anhydrous dichloromethane, add 0.144g (2.4mmol) acetic anhydride, add DMAP 29mg (0.24mmol) under stirring, stir at room temperature for 5h, TLC The detection response is complete. The reaction solution was washed three times with saturated sodium bicarbonate, dilute hydrochloric acid, and saturated brine, dried over anhydrous sodium sulfate, and the solvent was recovered to obtain a light yellow solid, which was recrystallized with chloroform-methanol and dried to obtain a light yellow solid with a yield of 80% %. 1 H NMR (400MHz, CDCl3) δ12.19 (s, 1H, 5-OH), 7.48-7.30 (m, 17H, Ar-H), 7.01 (d, J=9.1Hz, 1H, 5'-H), 6.48(d, J=2.2Hz, 1H, 8-H), 6.45(d, J=2.2Hz, 1H, 6-H), 5.25(s, 2H, OCH2C6H5), 5.21(s, 2H, OCH2C6H5), 5.13(s, 2H, OCH2C6H5), 2.25(s, 3H, CH3CO-). 13 C NMR(101MHz,C...
Embodiment 2
[0040] Embodiment 2: the preparation of quercetin-3-O-propionate (3b)
[0041] Synthesis of 7,3`,4`-O-tribenzylquercetin-3-O-acrylate
[0042] Weigh 1.144g (2mmol) tribenzyl quercetin, dissolve in 40ml anhydrous dichloromethane, add 0.173g (2.4mmol) acrylic acid, add DMAP 29mg (0.24mmol) under stirring, weigh 0.496g (2.4mmol) ) DCC was dissolved in anhydrous dichloromethane, and the dichloromethane solution of DCC was slowly added dropwise under ice-bath conditions. After the dropwise addition, the temperature was slowly raised to room temperature, and the stirring reaction was continued for 5 h. TLC detected that the reaction was complete. The reaction solution was refrigerated for 1 h, and the generated DCU was removed by filtration. The filtrate was washed three times with saturated sodium bicarbonate, dilute hydrochloric acid, and saturated brine, dried over anhydrous sodium sulfate, and the solvent was recovered to obtain a light yellow solid, which was recrystallized wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com