Bionic specific immunity adsorbing material and preparation method as well as application thereof
An immunoadsorbent material and a specific technology, applied in the field of biomimetic specific immunoadsorbent material for blood purification and its preparation, can solve the problems of cumbersome activation steps, lack of high selectivity and high efficiency, low reaction selectivity and the like, Achieve mild reaction conditions, improve coupling effect, and avoid side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
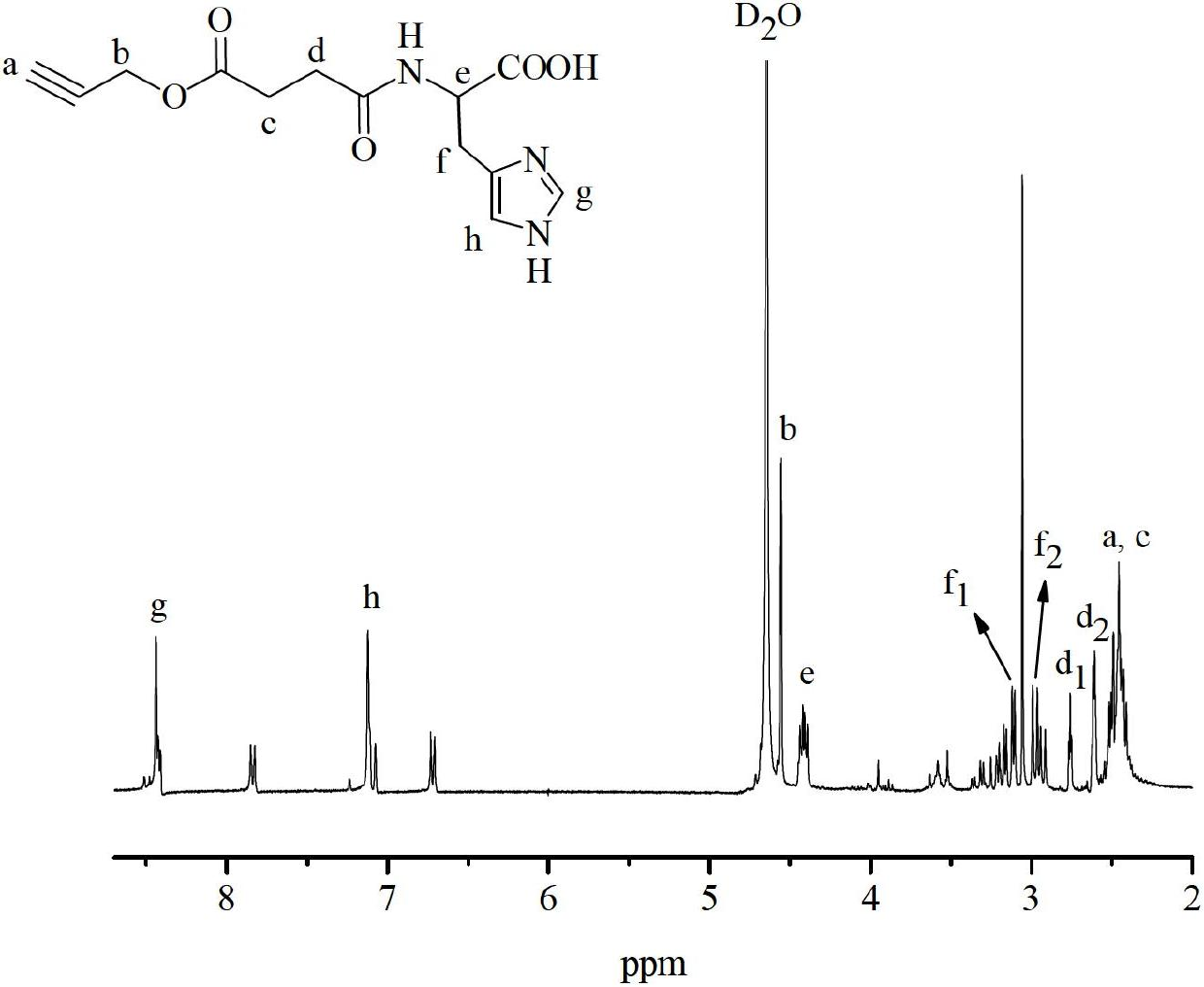
Embodiment 1
[0058] Preparation of Azide Sepharose Gels
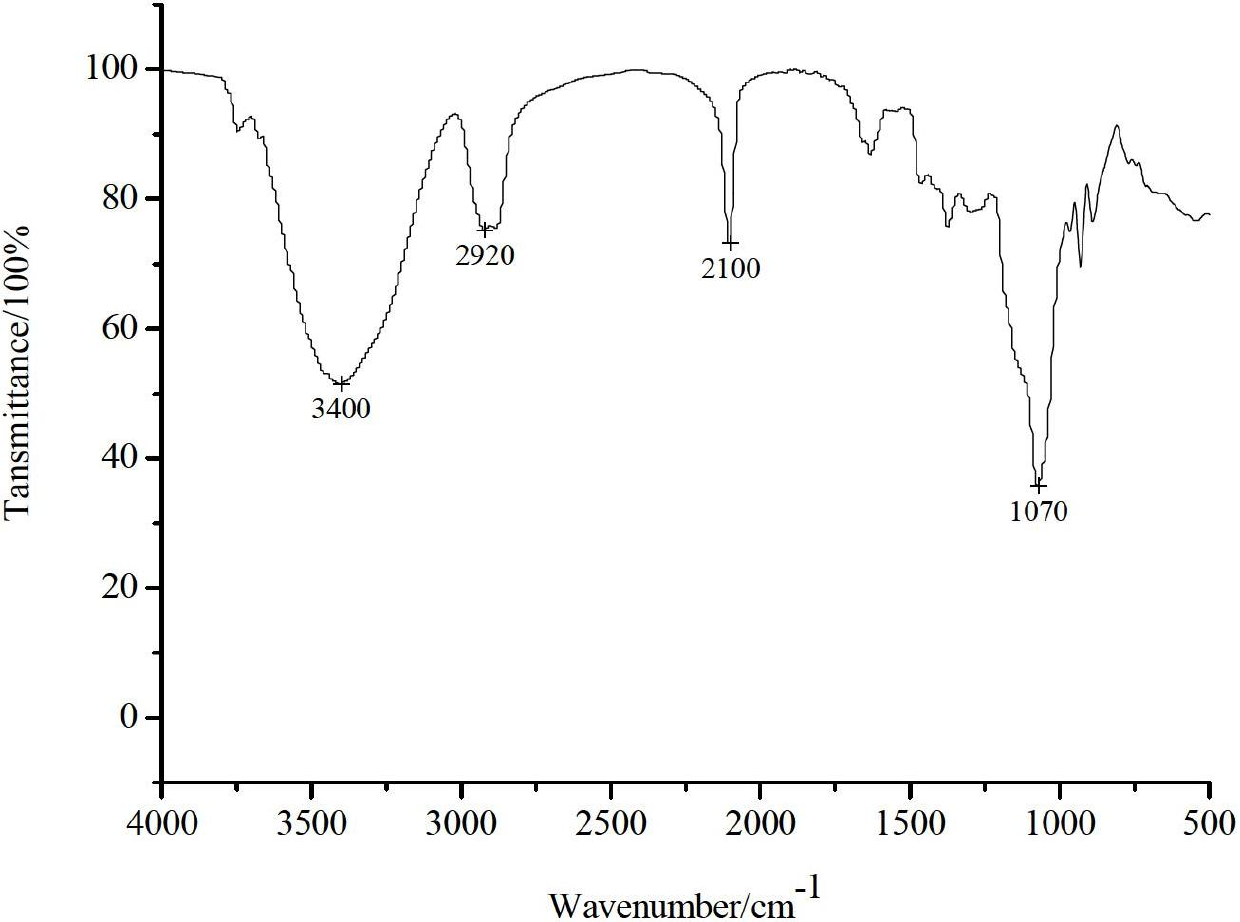
[0059] Add 4.0 g of drained agarose gel, 8 mL of epichlorohydrin and 4 mL of 3M sodium hydroxide solution into a 100 mL Erlenmeyer flask. After the mixture was stirred and reacted at room temperature for 3 h, it was washed with water and drained until the phenolphthalein did not turn red after adding 1.3M sodium thiosulfate to the washing liquid, and the epoxidized agarose was obtained. Dissolve 1.82g of sodium azide in 15mL of water, then add 4.0g of epoxidized agarose, and react at 20°C for 60h. After the reaction was completed, the gel was washed and dried to obtain an azide agarose gel. attached figure 1 The infrared spectrum shown is at 2100cm -1 The characteristic absorption peak of the azido group appeared at , which proved the existence of the azido group in the azide agarose.
[0060] Synthesis of Alkynoic Acids
[0061] 10.0 g of succinic anhydride, 1.01 g of triethylamine, 0.12 g of 4-dimethylaminopyridine and 50 mL ...
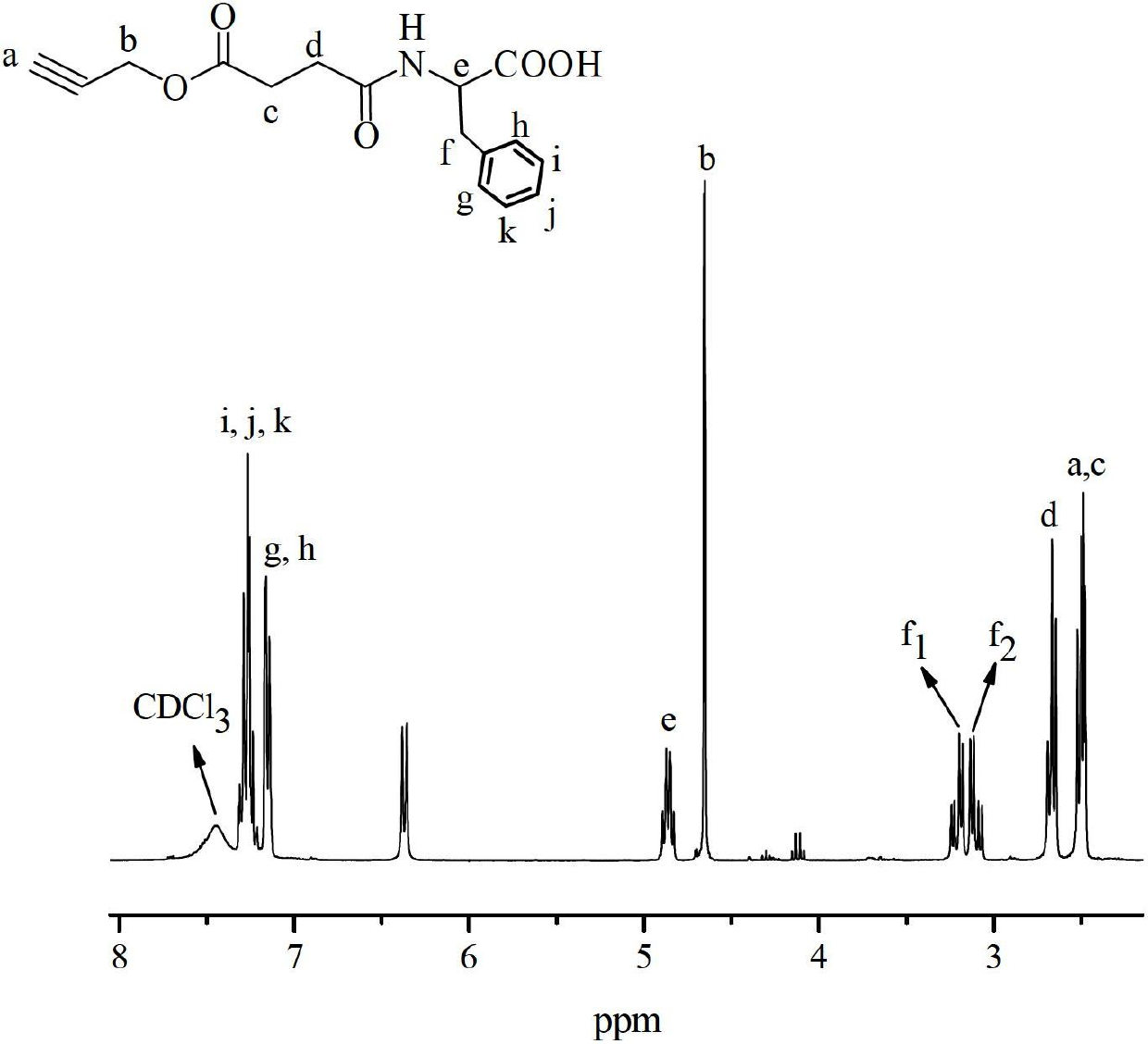
Embodiment 2
[0075] Preparation of Azide Sepharose Gels
[0076] Add 4.0 g of drained agarose gel, 8 mL of epichlorohydrin and 8 mL of 1.5 M sodium hydroxide solution into a 100 mL Erlenmeyer flask. After the mixture was stirred and reacted at room temperature for 4 h, it was washed with water and drained until the phenolphthalein did not turn red after adding 1.3M sodium thiosulfate to the washing liquid, thus obtaining epoxidized agarose. Dissolve 0.78g of sodium azide in 10mL of water, then add 4.0g of epoxidized agarose, and react at 40°C for 30h. After the reaction was completed, the gel was washed and dried to obtain an azide agarose gel.
[0077] Synthesis of Alkynoic Acids
[0078] 10.0 g of succinic anhydride, 10.1 g of triethylamine, 1.22 g of 4-dimethylaminopyridine and 250 mL of dichloromethane were placed in a 200 mL round bottom flask. At 10°C, 20 mL of a dichloromethane solution containing 16.8 g of propargyl alcohol was added dropwise. After the dropwise addition, the m...
Embodiment 3
[0092] Preparation of Azide Sepharose Gels
[0093] Add 4.0 g of drained agarose gel, 8 mL of epichlorohydrin and 6 mL of 2.5 M sodium hydroxide solution into a 100 mL Erlenmeyer flask. After the mixture was stirred and reacted at room temperature for 3.5 h, it was washed with water and drained until the phenolphthalein did not turn red after adding 1.3M sodium thiosulfate to the washing liquid, and the epoxidized agarose was obtained. Dissolve 3.9g of sodium azide in 25mL of water, then add 4.0g of epoxidized agarose, and react at 60°C for 6h. After the reaction was completed, the gel was washed and dried to obtain an azide agarose gel.
[0094] Synthesis of Alkynoic Acids
[0095] 10.0 g of succinic anhydride, 5.05 g of triethylamine, 0.61 g of 4-dimethylaminopyridine and 150 mL of dichloromethane were placed in a 200 mL round bottom flask. At 0°C, 15 mL of a dichloromethane solution containing 11.2 g of propargyl alcohol was added dropwise. After the dropwise addition, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com