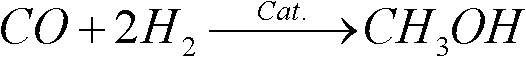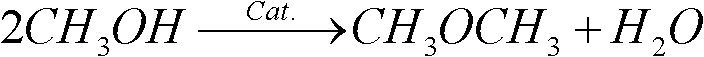Catalyst for preparing dimethyl ether by methanol and preparation method thereof
A technology of dimethyl ether and catalyst, applied in the field of preparation of the catalyst, can solve the problems of easy carbon deposition, reduce dimethyl ether, catalyst decline, etc., and achieve the effects of preventing activity decline, improving stability, and preventing carbon deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
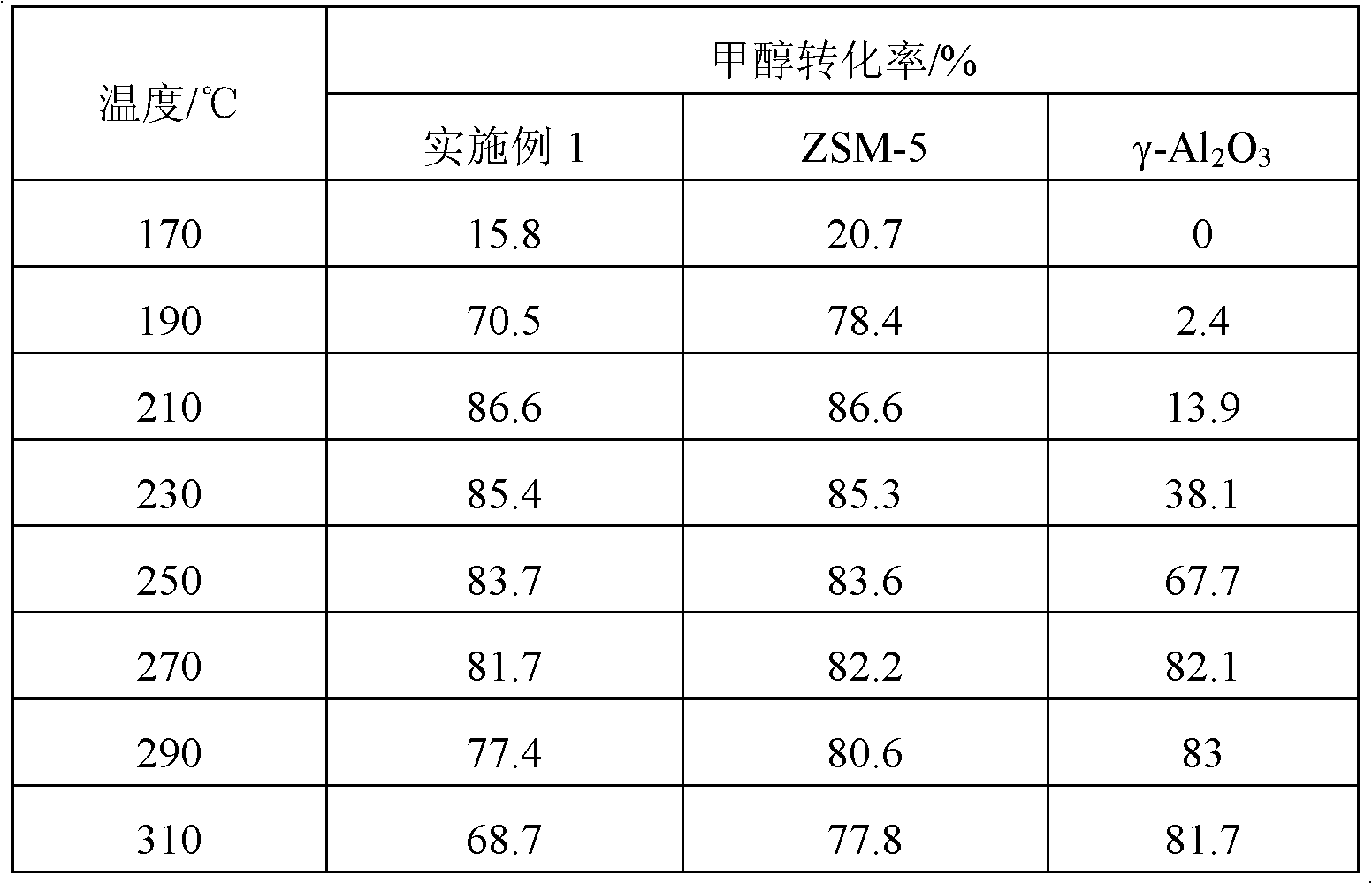
Embodiment 1
[0026] The catalyst for the production of dimethyl ether from methanol of the present embodiment is prepared as follows:
[0027] 1) Weigh 1 g of cetyltrimethylammonium bromide and add it to 50 mL of 1.5 mol / L KOH solution, then add 10 g of ZSM-5 zeolite to the above solution and stir for 45 minutes to obtain a mixed solution, wherein ZSM-5 zeolite for SiO 2 and Al 2 o 3 Composition, the molar ratio of Si and Al is 25:1;
[0028] 2) Transfer the above mixed slurry into a hydrothermal kettle, and carry out crystallization at 110°C for 24 hours. After crystallization and quenching, a primary crystallization mixed solution is obtained, and hydrochloric acid is added to adjust the pH value of the primary crystallization mixed solution to 8 Finally, put it into an autoclave for secondary crystallization at 110°C for 24 hours, quench after secondary crystallization to obtain a secondary crystallization mixture, filter the mixture, wash, and dry to obtain a solid powder;
[0029]...
Embodiment 2
[0031] The catalyst for the production of dimethyl ether from methanol of the present embodiment is prepared as follows:
[0032] 1) Weigh 1 g of tetradecyltrimethylammonium bromide and add it to 50 mL of 3 mol / L KOH solution, then add 5 g of mordenite to the above solution and stir for 20 minutes to obtain a mixed solution, wherein the mordenite is SiO 2 and Al 2 o 3 Composition, the molar ratio of Si and Al is 50:1;
[0033] 2) Transfer the above mixed slurry into a hydrothermal kettle, and carry out crystallization at 105°C for 6 hours. After crystallization and quenching, the primary crystallization mixed solution is obtained, and hydrochloric acid is added to adjust the pH value of the primary crystallization mixed solution to 6 Finally, put it into an autoclave for secondary crystallization at 105°C for 12 hours, quench after secondary crystallization to obtain a secondary crystallization mixture, filter the mixture with suction, wash, and dry to obtain a solid powder;...
Embodiment 3
[0036] The catalyst for the production of dimethyl ether from methanol of the present embodiment is prepared as follows:
[0037] 1) Weigh 1 g of octadecyltrimethylammonium bromide and add it to 50 mL of 1.5 mol / L KOH solution, then add 20 g of ZSM-35 zeolite to the above solution and stir for 35 minutes to obtain a mixed solution, wherein ZSM-35 zeolite for SiO 2 and Al 2 o 3 Composition, the molar ratio of Si and Al is 15:1;
[0038]2) Transfer the above mixed slurry into a hydrothermal kettle, and carry out crystallization at 130°C for 12 hours. After crystallization and quenching, a primary crystallization mixed solution is obtained, and hydrochloric acid is added to adjust the pH value of the primary crystallization mixed solution to 11 Finally, put it into an autoclave for secondary crystallization at 130°C for 18 hours, quench after secondary crystallization to obtain a secondary crystallization mixture, filter the mixture, wash, and dry to obtain a solid powder;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com