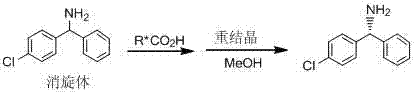
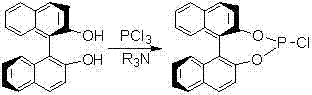
Method for preparing chiral diaryl- substitute methylamine
A technology of diaryl and aryl formaldehyde, which is applied in the field of preparation of chiral diaryl substituted methylamine, can solve the problems of low yield and high cost of raw and auxiliary materials, achieve high yield, reduce production cost and reduce emissions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
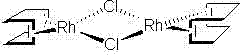
[0039] Embodiment 1: Preparation of (1,5-cyclooctadiene) rhodium chloride dimer
[0040] In a 250 ml dry three-necked round-bottom flask, add an electromagnetic stirrer and 60 ml of absolute ethanol, pass high-purity nitrogen gas for 20 minutes to release the oxygen dissolved in the ethanol, and then add 10 g (0.038 mol) rhodium trichloride Trihydrate, 8 g (0.075 mol) of anhydrous sodium carbonate and 5.7 g (0.053 mol) of 1,5-cyclooctadiene, the temperature was raised to 55~60°C, and the reaction was kept for 8 hours, and the conversion was complete. Remove the inorganic salt by filtration, dry the organic phase and evaporate to dryness to obtain a yellow solid, which is recrystallized in isopropanol to obtain 10.6 g of an orange solid, which is the product (1,5-cyclooctadiene) rhodium chloride dimer . The yield based on rhodium trichloride trihydrate was 82.8%.
Embodiment 2
[0041] Embodiment 2: Preparation of (1,5-cyclooctadiene) rhodium chloride dimer
[0042]In a 100 ml dry three-neck round-bottom flask, add an electromagnetic stirrer and 30 ml of anhydrous methanol, pass through argon for 20 minutes to release the oxygen dissolved in methanol, and then add 5 g (0.019 mol) of rhodium trichloride Hydrate, 5.25 g (0.038 mol) of anhydrous potassium carbonate and 10.2 g (0.094 mol) of 1,5-cyclooctadiene, the temperature was raised to 65°C, and the reaction was refluxed for 12 hours, and the conversion was complete. Remove the inorganic salt by filtration, dry the organic phase and evaporate to dryness to obtain a yellow solid, which is recrystallized in isopropanol to obtain 5.4 g of an orange solid, which is the product (1,5-cyclooctadiene) rhodium chloride dimer . The yield based on rhodium trichloride trihydrate was 84.5%.
Embodiment 3
[0043] Embodiment 3: Preparation of (1,5-cyclooctadiene) rhodium chloride dimer
[0044] Other conditions are the same as in Example 2, except that the amount of 1,5-cyclooctadiene is 2.04 g (0.019 mol), and 4.9 g of the product (1,5-cyclooctadiene) rhodium chloride dimerization is obtained as an orange solid body. The yield based on rhodium trichloride trihydrate is 76.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com