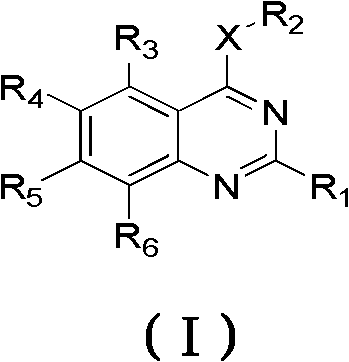
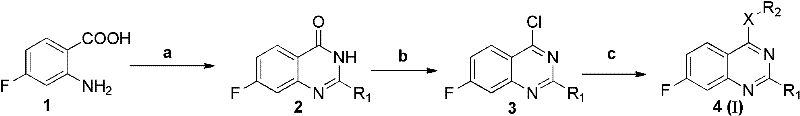
2-substituent-7-fluorine-4-aromatic mixed base quinazoline derivant and preparation method and use thereof
A technology of fluoroquinazoline and its derivatives, which is applied in the field of medicine and can solve problems such as difficult resection, short survival period of tumors, and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
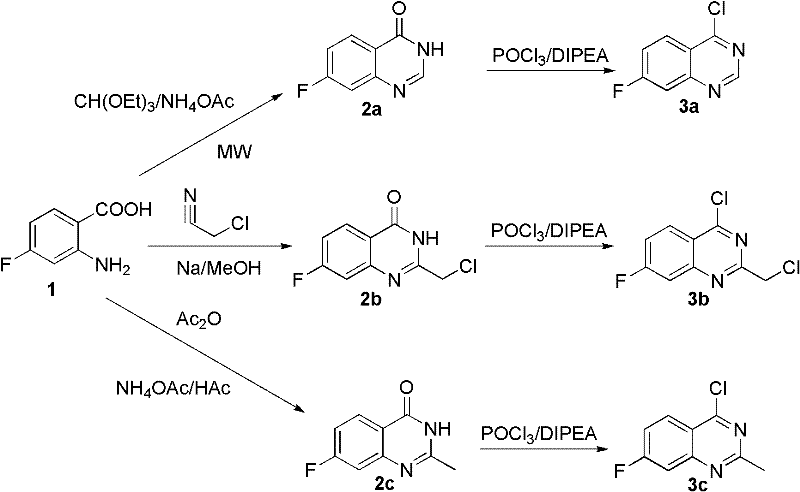
[0019] The preparation of experimental example 1 key intermediate 4-chloro-7-fluoroquinazoline 3a
[0020]
[0021] a) 4-Fluoroanthranilic acid 1 (1.55g, 10mmol), triethyl orthoformate (2.16mL, 13mmol) and ammonium acetate (1g, 13mmol) were uniformly mixed together and reacted under microwave irradiation (210W) After 5 minutes, cool to room temperature, filter, wash the filter cake with water, and recrystallize with ethanol to obtain 0.75 g of white solid as 7-fluoro-4(3H)quinazolinone 2a. Yield 46%.
[0022] 1 H-NMR (400MHz, DMSO-d 6 ): δ12.52 (br s, 1H), 8.18 (dd, J=2.4, 8.0Hz, 2H), 7.48 (dd, J=2.0, 8.0Hz, 1H), 7.42 (dd, J=2.4, 8.0Hz , 1H).
[0023] ESI-MS: m / z 183.03 (M+H + ).
[0024] b) Suspend the 7-fluoro-4(3H)quinazolinone solid 2a (0.50g, 3mmol) obtained above and N,N-diisopropylethylamine (DIPEA, 1.57mL, 9mmol) in 15mL In anhydrous toluene, heat to reflux for one hour, cool to room temperature, add POCl to the reaction solution 3 (0.82mL, 9mmol), heated to...
experiment example 2
[0025] Experimental example 2 Preparation of key intermediate 2-chloromethyl-4-chloro-7-fluoroquinazoline 3b
[0026]
[0027] a) Sodium metal (0.025g) was added to anhydrous methanol (5mL), stirred under nitrogen protection until the reaction was complete, chloroacetonitrile (1.125g, 15mmol) was added dropwise to the above reaction solution, and after stirring at room temperature for 40 minutes, Then add 4-fluoroanthranilic acid 1 (0.776g, 5mmol) in methanol solution (25mL), the whole reaction system was stirred at room temperature under the protection of nitrogen for 2 hours, the pH was adjusted to 6-7 with dilute hydrochloric acid, and the solid was precipitated by suction filtration , washed the filter cake with cold methanol, and dried to obtain 0.778 g of white solid 2-chloromethyl-7-fluoro-4(3H)quinazolinone 2b, yield 72%.
[0028] 1 H-NMR (400MHz, DMSO-d 6 ): δ12.72 (br s, 1H), 8.18 (dd, J=2.4, 8.0Hz, 1H), 7.48 (dd, J=2.0, 8.0Hz, 1H), 7.42 (dd, J=2.4, 8.0Hz , 1H)...
experiment example 3
[0032] Experimental Example 3 Preparation of key intermediate 2-methyl-4-chloro-7-fluoroquinazoline 3c
[0033]
[0034] a) Dissolve 4-fluoro-2-aminobenzoic acid 1 (1.55g, 10mmol) in acetic anhydride (20ml, 200mmol), heat to reflux, react for 2 hours under nitrogen protection, cool to room temperature, and filter with suction A solid was precipitated, and the filter cake was washed with ether and dried to obtain 1 g of benzoxazinone intermediate with a yield of 56%.
[0035] b) Heat the benzoxazinone intermediate (1g, 5.58mmol) and ammonium acetate (1.5g, 19mmol) synthesized above to reflux, add 5mL of acetic acid, react until complete, cool to 50°C, add 8mL of methanol, and heat To reflux, react for 45 minutes, cool to room temperature, filter out the solid with suction, wash the filter cake with methanol to obtain 0.54 g of white solid 2-methyl-7-fluoro-4(3H)quinazolinone 2c, yield 54% .
[0036] c) Suspend the 2-methyl-7-fluoro-4(3H)quinazolinone solid (0.54g, 3mmol) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com