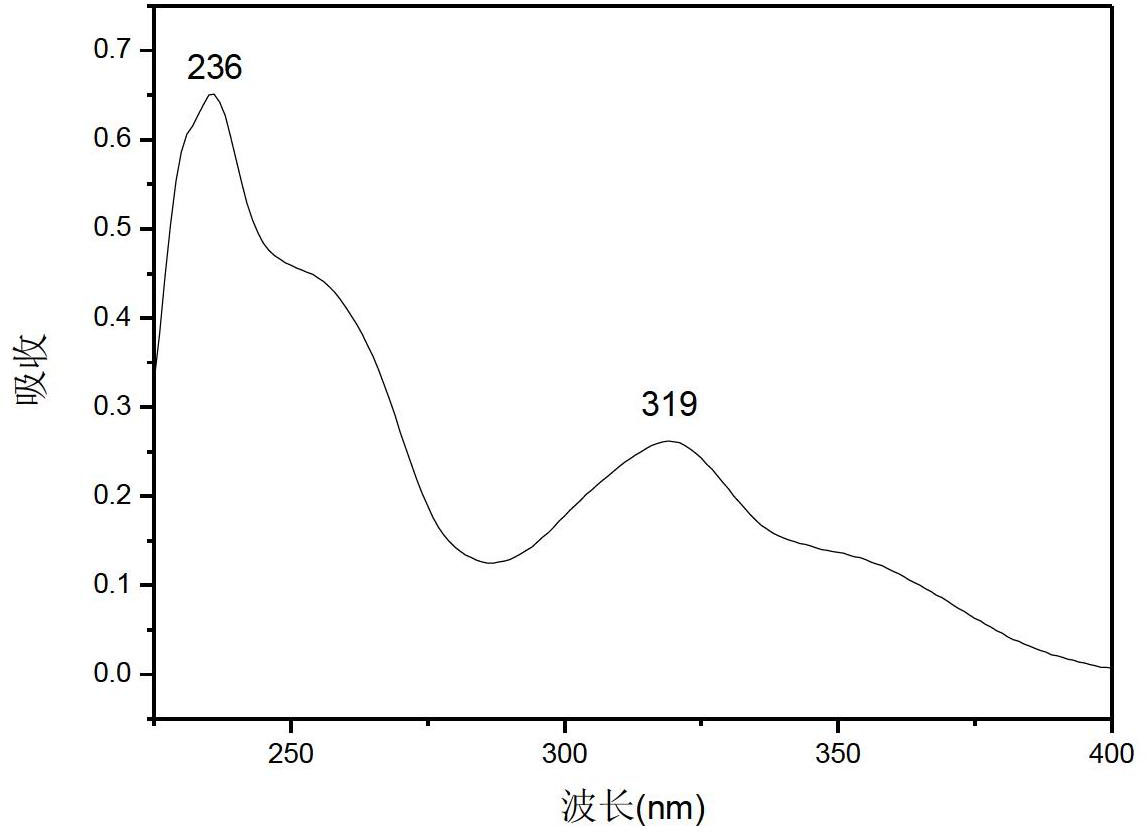
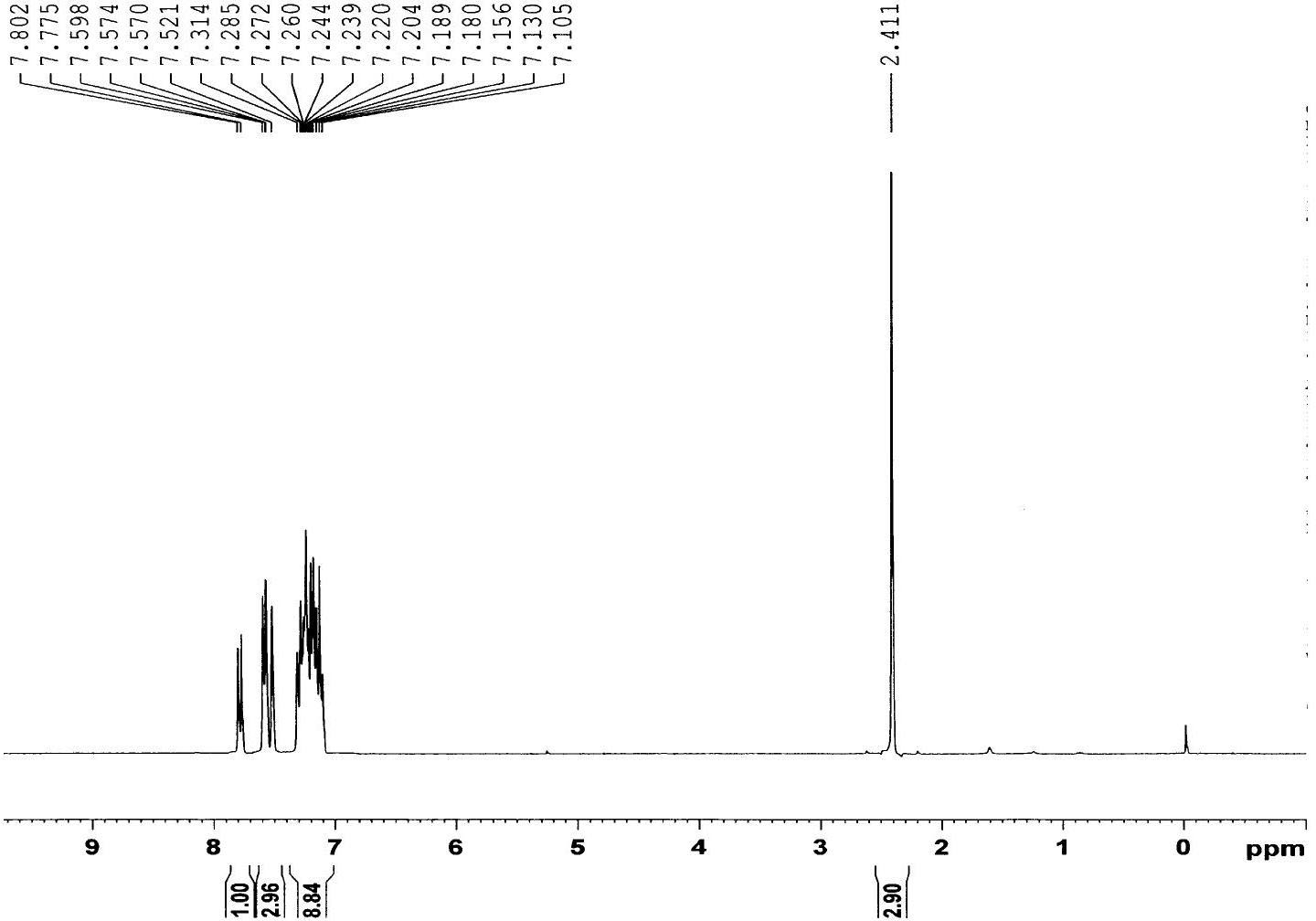
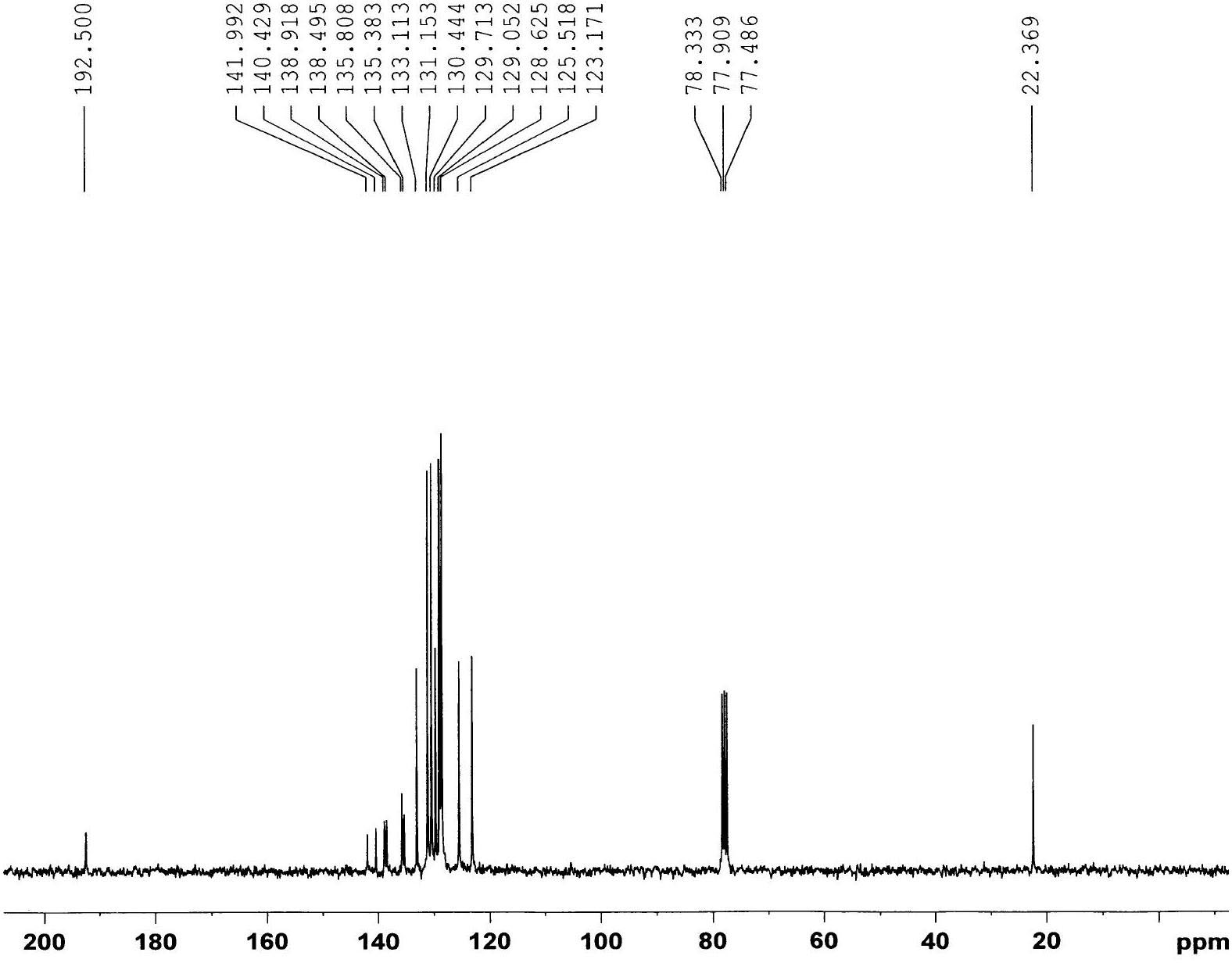
Phenyl benzothiophene ketone compound and preparation method and application thereof
A technology of thiophenemethone and phenylbenzene, which is applied in the application field of phenylbenzothiophenemethone compound and its preparation, and UV-curable coatings, and can solve the problems of odor, poisonous gas, and low content of active ingredients in the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Put a magnetic stirrer in a three-necked flask equipped with a constant pressure dropping funnel and a condenser tube (with a drying tube, which is then connected to a gas absorption device), and sequentially add 5-methyl-3-phenylbenzothiophene 2.24g (10mmol), CH 2 Cl 2 (30ml), 1.74g (13mmol) of anhydrous aluminum trichloride was added to the reactor, and after stirring evenly, 1.68g (12mmol) of benzoyl chloride was slowly added dropwise under ice bath conditions. After the dropwise addition was completed, return to room temperature and react Monitor with TLC; After the reaction finishes, put the above mixture into ice water (100ml), add concentrated hydrochloric acid (10ml) dropwise to carry out acidolysis, stir, after the solid in the mixture dissolves completely, transfer to a separatory funnel, separate the organic layer; the organic layer was washed once with an equal volume of sodium hydroxide solution and water, dried, filtered, spin-dried, and separated by colu...
Embodiment 2
[0024] Put a magnetic stirrer in a three-necked flask equipped with a constant pressure dropping funnel and a condenser tube (with a drying tube, which is then connected to a gas absorption device), and sequentially add 5-methyl-3-phenylbenzothiophene 2.24g (10mmol), CH 2 Cl 2 (30ml), 1.47g (11mmol) of anhydrous aluminum trichloride was added to the reactor, and after stirring evenly, 1.68g (12mmol) of benzoyl chloride was slowly added dropwise under ice bath conditions. After the dropwise addition was completed, return to room temperature and react Monitor with TLC; After the reaction finishes, put the above mixture into ice water (100ml), add concentrated hydrochloric acid (10ml) dropwise to carry out acidolysis, stir, after the solid in the mixture dissolves completely, transfer to a separatory funnel, separate the organic layer; the organic layer was washed once with an equal volume of sodium hydroxide solution and water, dried, filtered, spin-dried, and separated by colu...
Embodiment 3
[0026] Put a magnetic stirrer in a three-necked flask equipped with a constant pressure dropping funnel and a condenser tube (with a drying tube, which is then connected to a gas absorption device), and sequentially add 5-methyl-3-phenylbenzothiophene 2.24g (10mmol), CH2Cl2 (30ml), 2.01g (15mmol) of anhydrous aluminum trichloride were added to the reactor, after stirring evenly, 1.68g (12mmol) of benzoyl chloride was slowly added dropwise under ice bath conditions, and the dropwise addition was completed Afterwards, it was returned to room temperature, and the reaction was monitored by TLC; after the reaction, the above mixture was placed in ice water (100ml), and concentrated hydrochloric acid (10ml) was added dropwise for acidolysis, stirred, and when the solid in the mixture was completely dissolved, it was transferred to A separatory funnel was used to separate the organic layer; the organic layer was washed once with an equal volume of sodium hydroxide solution and water, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com