Method for extracting and purifying phloretin from Malus toringoides(Rehd.) Hughes. and Malus tiansitoria(Batal.)Schneid.
A purification method and technology of phloretin are applied in the field of medicine to achieve the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 The separation and purification method of phloretin of the present invention
[0022] (1) Take 8kg of dried leaves of Begonia variegata, crush or pulverize by hand, weigh about 1kg each time, add 5 times of 95% ethanol for reflux extraction, extract twice, filter, collect the subsequent filtrate, and recover under reduced pressure to obtain Ethanol fraction extract 563g. The extract of the ethanol part was dissolved in water, and then 2 times of ethyl acetate was added for repeated extraction until the ethyl acetate solution was colorless, and the ethyl acetate solutions were combined and recovered under reduced pressure to obtain 240 g of the extract of the ethyl acetate part.
[0023] (2) Add an appropriate amount of methanol to the ethyl acetate part to dissolve, and at the same time add 200g of silica gel (200-300 mesh) to mix the sample. Take 3000g of silica gel for wet packing, add the sample after the silica gel column is evenly precipitated, use ch...
Embodiment 2 Embodiment 1
[0026] The structure identification of the product obtained in Example 2 Example 1
[0027] Get the phloretin obtained in Example 1 and carry out structural identification, and the data are as follows:
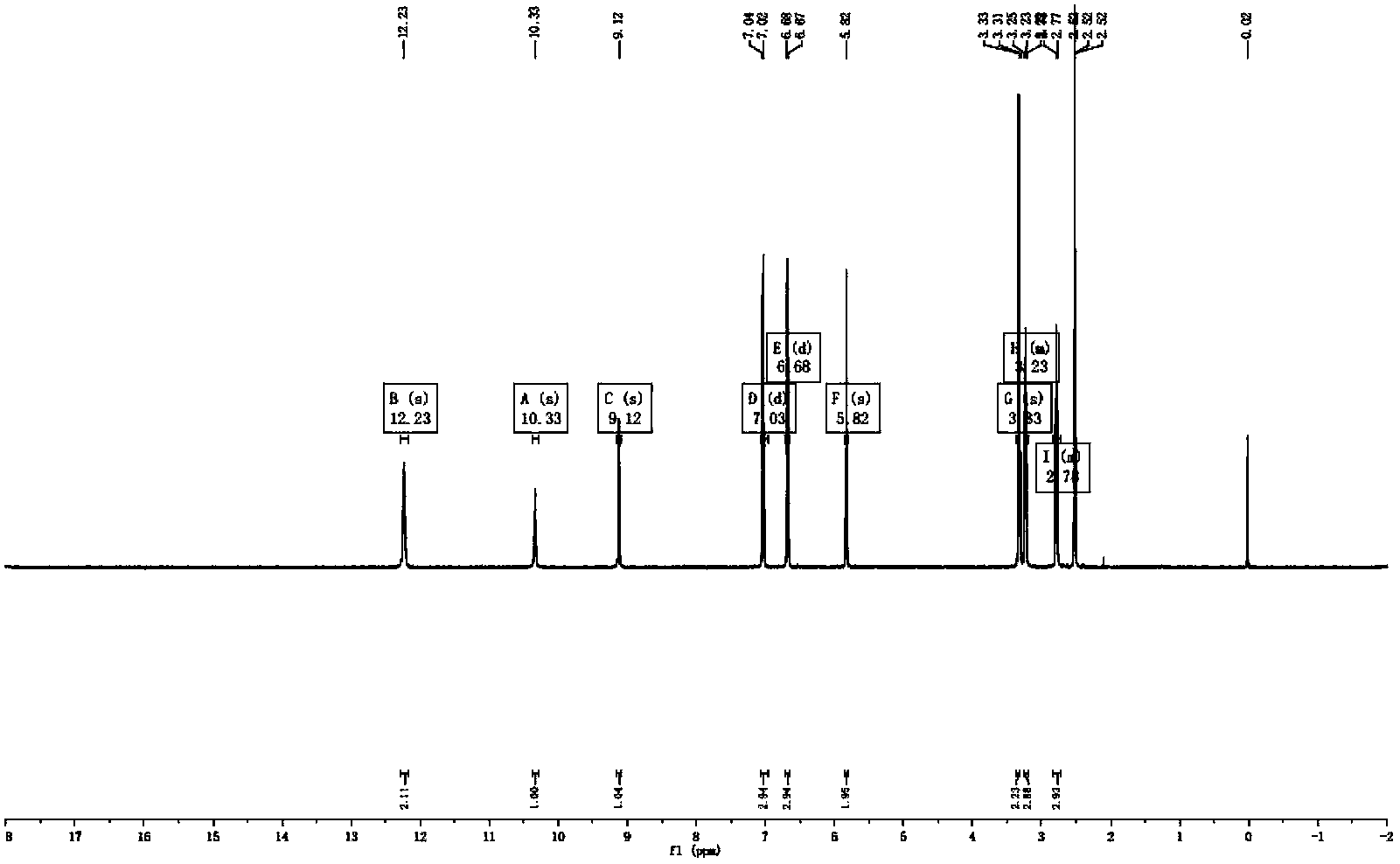
[0028] This product is a light pink powder (methanol), which is bright blue when sprayed with aluminum trichloride color developer under TLC ultraviolet lamp. The reaction of magnesium hydrochloride powder was positive, and it was preliminarily judged to be flavonoids or flavonols. Molecular formula: C 15 H 14 O 5
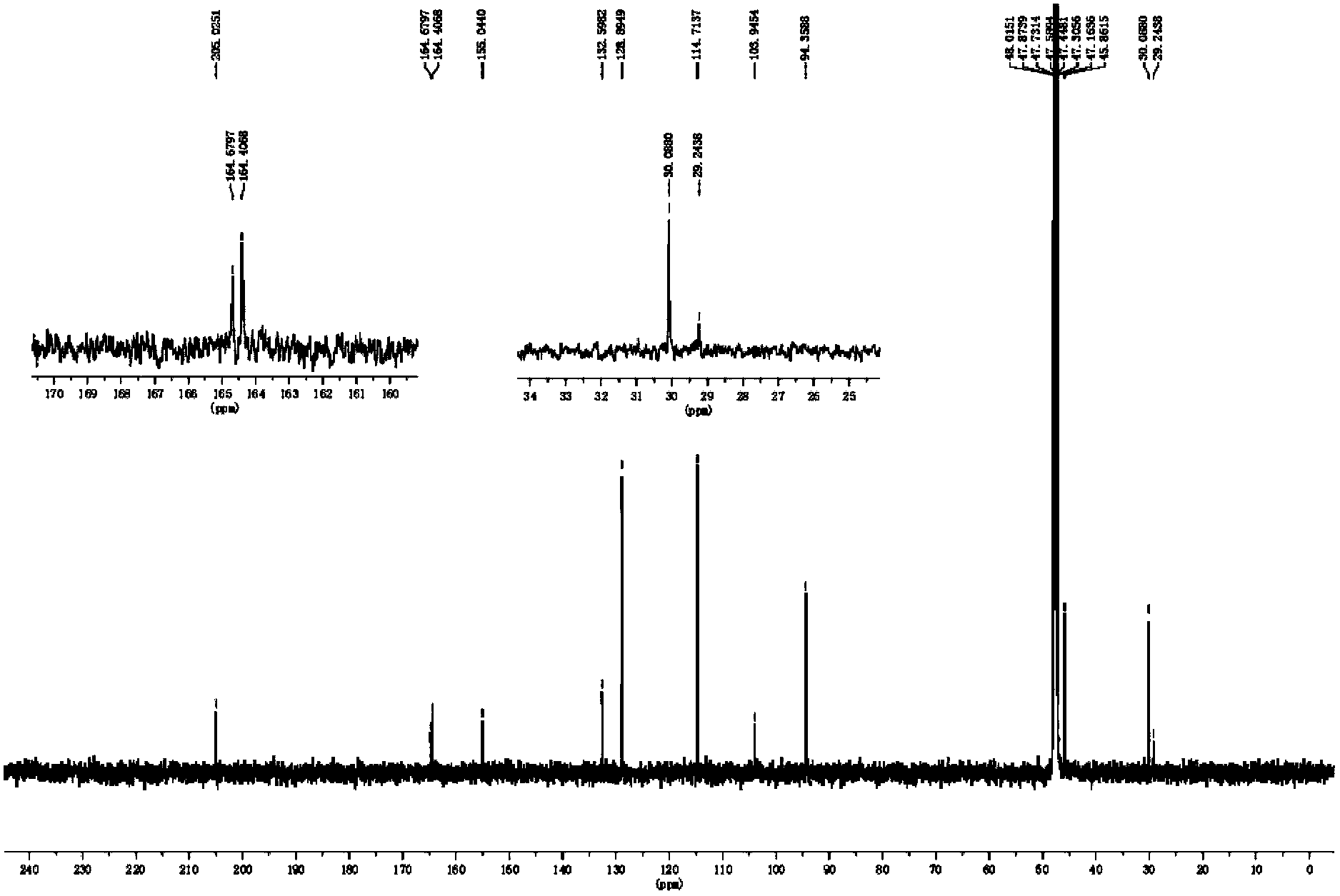
[0029] 1H-NMR (600MHz, DMSO-d612.23(2H,s,2',6'-OH), 10.33(1H,s,4'-OH), 9.12(1H,s,4-OH), 7.03( 2H,s,H-3',5'),6.68(2H,d,J=8.5Hz),5.82(2H,s,H-3',5'),3.23(2H,d,J=7.6Hz ),2.78(2H,d,J=7.6Hz),13C-NMR(151MHz,DMSO-d6)205.0(C=O),164.7(C-4'),164.4(C-2',C-6' ),155.0(C-4),132.5(C-1),128.9(C-2,C-6),114.7(C-3,C-5)103.9(C-1')94.4(C-3' ,C-5'), 45.9(C-α), 30.0(C-β) were identified as the compound Phloretin by comparison with literature data.
[0030]
Embodiment 3
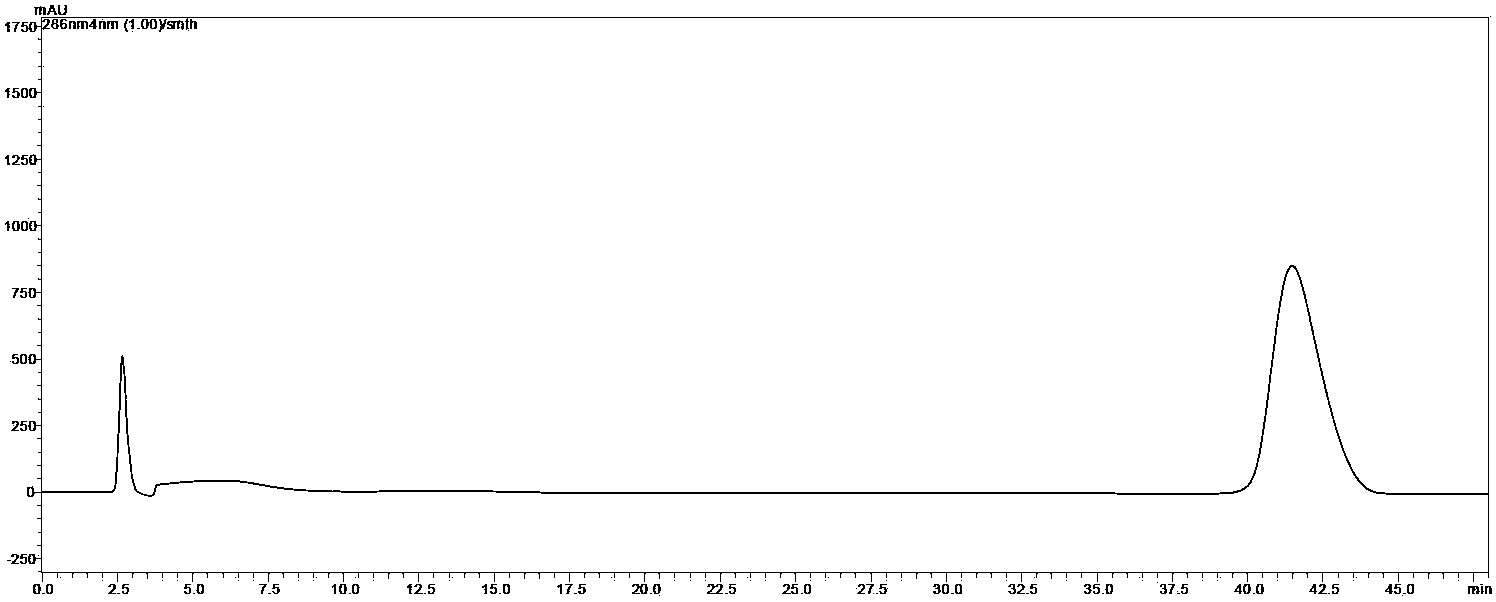
[0031] Example 3 Purity analysis of phloretin prepared by the method of the present invention
[0032] The purity of the phloretin prepared in Example 1 was detected, and the conditions were as follows:
[0033] Instrument: Agilent1200 high performance liquid chromatograph, DiamonsilTM C18HPLC chromatographic column (250×4.6mm, 5μm), BP211D electronic analytical balance (1 / 100,000, Sartorius Germany Sartorius AG).
[0034] Mobile phase: 27% acetonitrile-water
[0035] Detection wavelength: 286nm
[0036] Temperature: 30 degrees Celsius
[0037] Injection volume: 20μl
[0038] Elution conditions: isocratic elution
[0039] result:
[0040] Measured by high performance liquid chromatography, the purity of the phloretin prepared by the method of the invention reaches 98.61%, and the recovery rate is 65.12%.
[0041] The method does not use enzymatic hydrolysis or acid hydrolysis, and for the first time, phloretin is isolated from plants in the Rosaceae apple genus Rowan app...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com