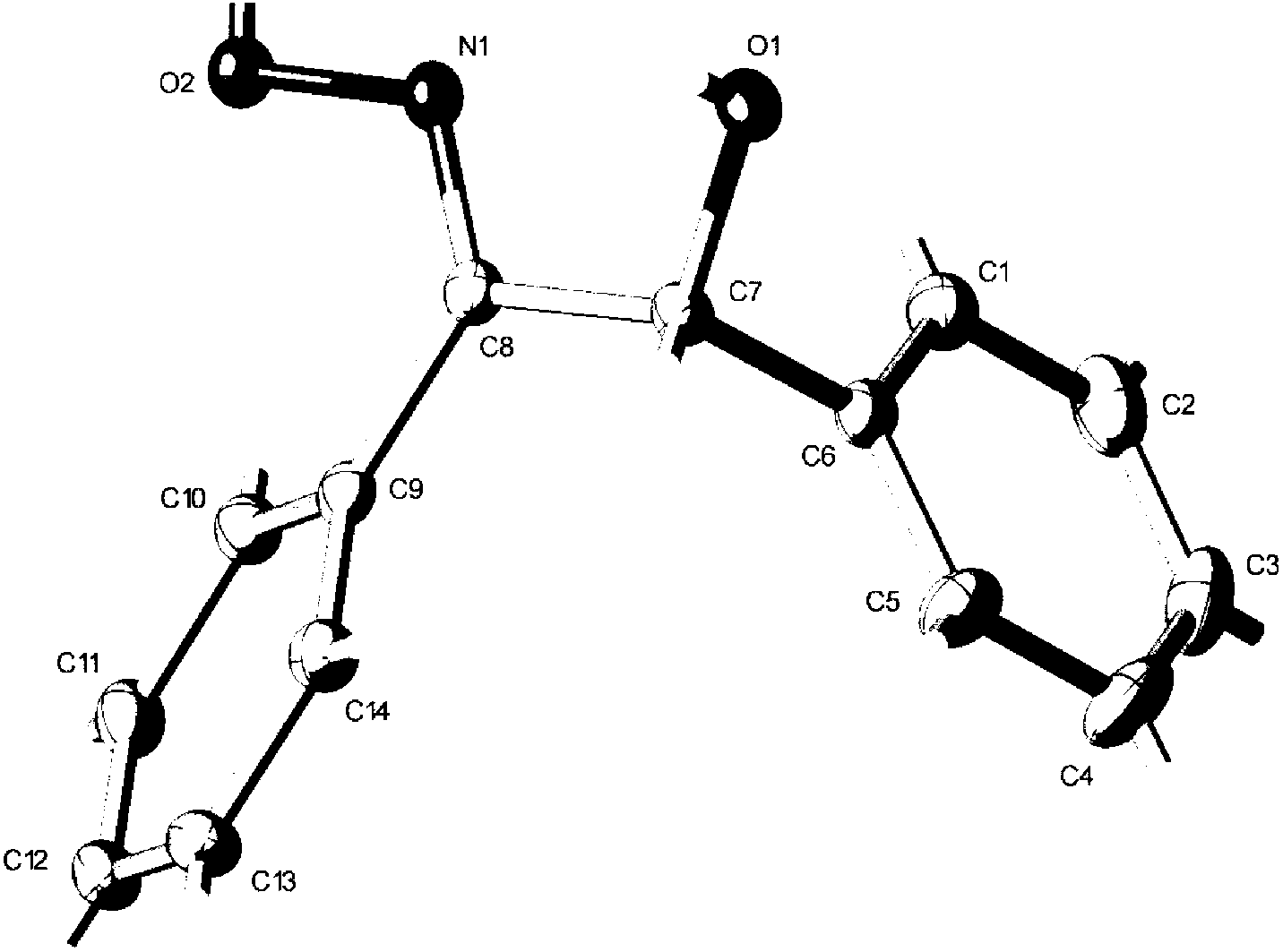
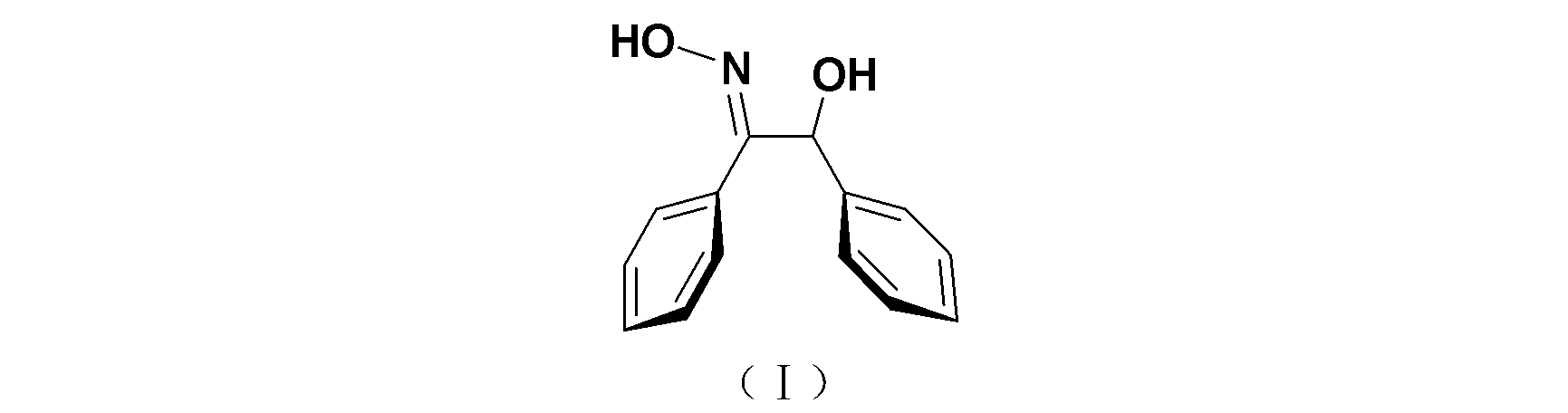
Synthesis method of trans-alpha-benzoinoxime
A technology of benzoin oxime and synthesis method, which is applied in the field of organic chemical synthesis, can solve problems such as low selectivity, reduced use efficiency, and difficult separation operation, and achieve the effect of simple product purification, easy implementation, and improved separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of α-benzoin
[0027] 5.3 g (50 mmol) of benzaldehyde, 0.23 g (0.5 mmol) of 1mol% imidazolium salt ionic liquid catalyst and 100 mL of tetrahydrofuran were sequentially added into a 250 mL three-necked flask, heated and stirred to 60 °C, and then 20 mol% sodium hydroxide 0.4 g (10 mmol), the reaction solution was heated to reflux for 8 hours, cooled to room temperature, added 50 mL of water, extracted three times with ethyl acetate, combined the organic phases, washed twice with saturated brine, dried the organic phase with anhydrous sodium sulfate, and filtered , the organic solvent was removed by a rotary evaporator to obtain a light yellow oil; 20 mL of absolute ethanol was added for recrystallization, cooled and filtered to obtain 2.4 g of white needle-shaped crystals, with a yield of 45%.
[0028] 1 H NMR (300 MHz, DMSO-d 6 ): δ 6.02~6.04 (m, 1H, OH), 6.08 (d, 1H, J = 6Hz ), 7.20~7.34 (m, 3H), 7.40~7.48 (m, 4H), 7.54~7.59 (m, 2H ), 7.98~8.01 (m, 2H...
Embodiment 2
[0033] (1) Synthesis of α-benzoin
[0034] 5.3 g (50 mmol) of benzaldehyde, 0.23 g (0.5 mmol) of 1mol% imidazolium salt ionic liquid catalyst and 100 mL of dichloromethane were successively added into a 250 mL three-necked flask, and 0.4 g (10 mmol), the reaction solution was stirred at room temperature for 12 h, added 50 mL of water, extracted three times with ethyl acetate, combined the organic phase, washed twice with saturated brine, dried the organic phase with anhydrous sodium sulfate, filtered, and the organic solvent was removed with a rotary evaporator , to obtain a light yellow oil; recrystallized from a mixed solution of absolute ethanol and petroleum ether to obtain 1.5 g of white needle-like crystals, with a yield of 28%.
[0035] (2) Synthesis of trans-α-benzoin oxime
[0036] Add 1.38 g (6.5 mmol) of α-benzoin, 0.5 g (7.2 mmol) of hydroxylamine hydrochloride and 20 mL of absolute ethanol into a 100 mL single-necked round bottom flask, stir at room temperature f...
Embodiment 3
[0038] (1) Synthesis of α-benzoin
[0039] Add 0.53 g (5 mmol) of benzaldehyde, 23 mg (0.05 mmol) of imidazolium salt ionic liquid catalyst, and 10 mL of tetrahydrofuran into a 50 mL three-necked flask in sequence, heat and stir to 60 °C, then add 40 mg (1 mmol) of sodium hydroxide ), the reaction solution was heated to reflux for 8 hours, cooled to room temperature, added 10 mL of water, extracted three times with ethyl acetate, combined the organic phase, washed twice with saturated brine, dried the organic phase with anhydrous sodium sulfate, filtered, and used for organic solvent Removed by rotary evaporator to obtain an oily substance; recrystallized from a mixed solution of ethyl acetate and petroleum ether to obtain 0.16 g of crystals, with a yield of 30%.
[0040] (2) Synthesis of trans-α-benzoin oxime
[0041] Add 0.15 g (0.7 mmol) of α-benzoin, 54 mg (0.78 mmol) of hydroxylamine hydrochloride and 10 mL of absolute ethanol into a 50 mL single-necked round bottom flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com