Imidazole ionic liquid with high alkali-resistant stability and application of imidazole ionic liquid
An ionic liquid and imidazole technology, applied in organic chemistry and other directions, can solve the problems of insufficient alkali resistance of anion exchange membranes, and achieve the effects of low cost, simple preparation process, and good alkali resistance and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
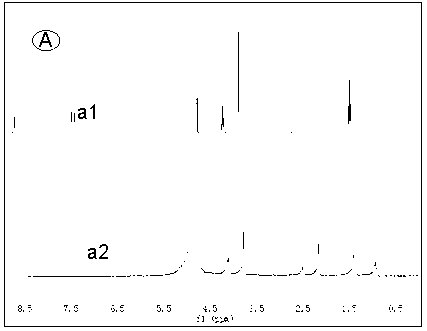
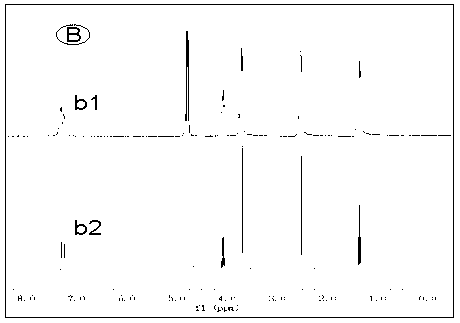
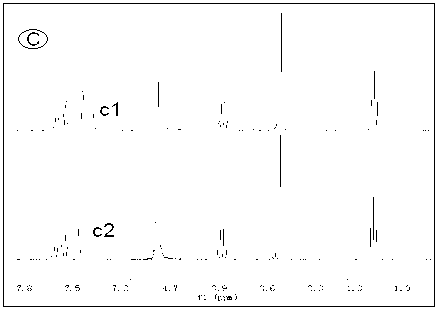
[0025] Alkali resistance test of several ionic liquids: put an appropriate amount of ionic liquid in a deuterated aqueous solution of 1M KOH at 80°C, use nuclear magnetic resonance technology to monitor the structural changes of the ionic liquid in real time, and evaluate by detecting the change of the H spectrum of the ionic liquid Alkali resistance of ionic liquids. Such as Figure 1 to Figure 4 As shown, the four groups of pictures A, B, C, and D respectively represent four kinds of ionic liquids , , , The H-spectrum of each figure, the upper spectral line is the original H-spectrum of the ionic liquid, and the lower spectral line is the H-spectrum after 60 hours of alkali resistance test. figure 1 After the ionic liquid was passed through natron, many miscellaneous peaks appeared in the NMR spectrum, indicating that the ionic liquid was not stable enough in 1M KOH solution at 80°C, and its structure changed. and figure 2 , image 3 , Figure 4 The spectra aft...
Embodiment 2
[0027] Polymerized Ionic Liquids Containing Substituent Groups at the 2-Position of Imidazole Ring Preparation of:
[0028] Under nitrogen protection, add 5.46g (0.05mol) 2-isopropylimidazole, 7.10g (0.05mol) methyl iodide and 5.61g (0.10mol) KOH to a three-necked flask with 40ml of acetonitrile and stir at room temperature for 4 hours , filtered, and rotary evaporated to obtain a crude product, which was dissolved in chloroform, extracted with water, and the chloroform layer was taken, and anhydrous magnesium sulfate was added to remove water, and the rotary evaporated to obtain 5.6 g of the product 1-methyl-2 isopropylimidazole (produced Rate: 90%).
[0029] Under nitrogen protection, 3.10 g (0.025 mol) of 1-methyl-2 isopropylimidazole, 3.82 g (0.025 mol) of vinylbenzyl chloride and 20 ml of ethyl acetate were added to the three-necked flask, and the reaction was carried out in an ice-water bath for 48 hours. The reactant was washed 3 times with ethyl acetate, and vacuum-...
Embodiment 3
[0032] 0.2g, styrene 0.1g, acrylonitrile 0.2g, 0.01g, 0.02g of divinylbenzene, mix the solution evenly, apply it on the mold, polymerize at 75°C for 6 hours, and form a film in situ. The anion exchange membrane was then soaked in 1M KOH solution at 60°C for 24 hours to convert anions into OH - , soaked in deionized water for 24 hours to remove the KOH remaining in the membrane, the obtained OH - type anion exchange membrane, the conductivity at room temperature is 1.14×10 -2 S cm -1 , with an ionic conductivity of 3.53×10 at 60 °C -2 S cm -1 . It is then subjected to an alkali resistance test: soak it to 80 o After 120 hours in 1M potassium hydroxide solution at C, soak in distilled water to remove the residual KOH in the film. The conductivity at room temperature is 1.21×10 -2 S cm -1 , with an ionic conductivity of 3.46×10 at 60 °C -2 S cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com