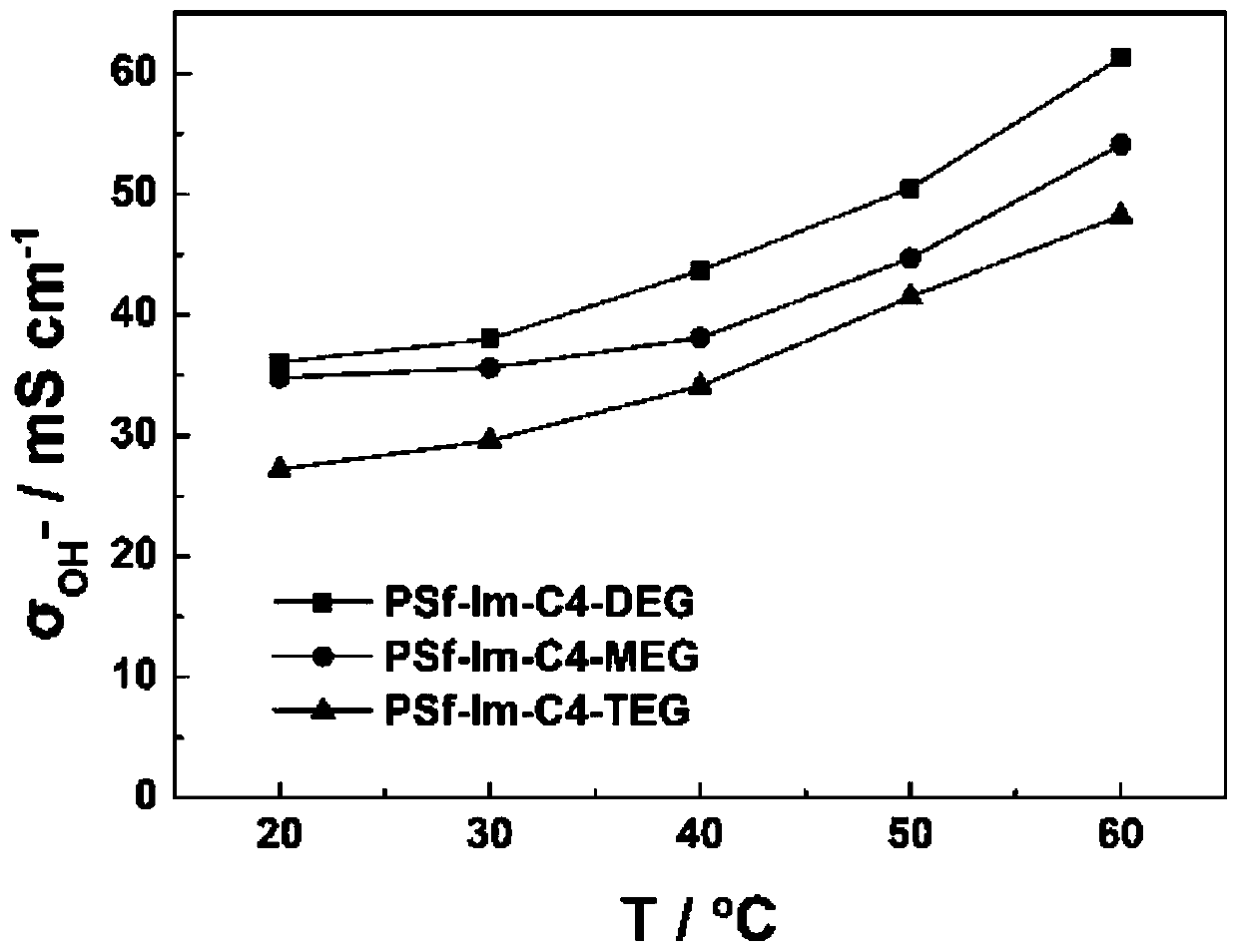
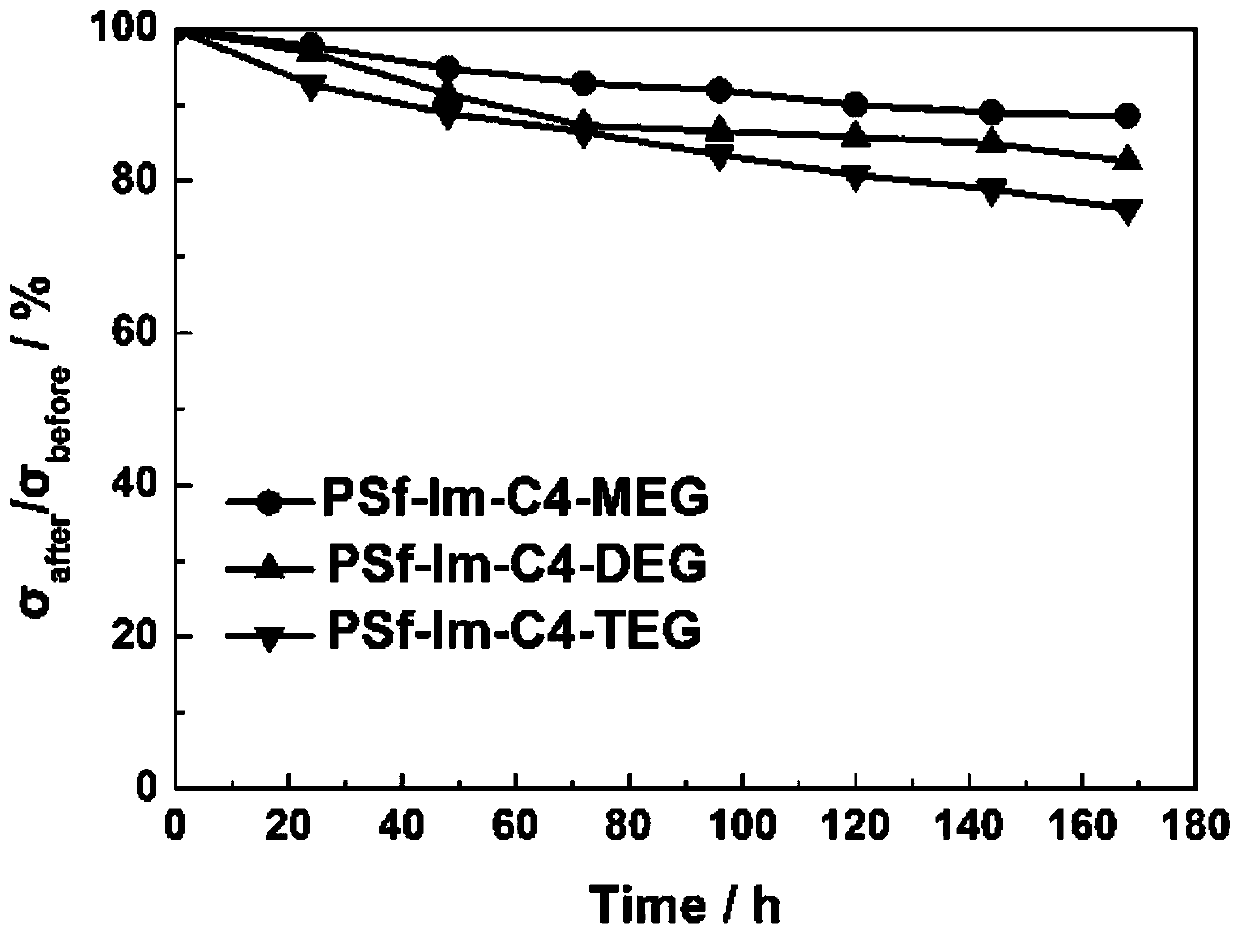
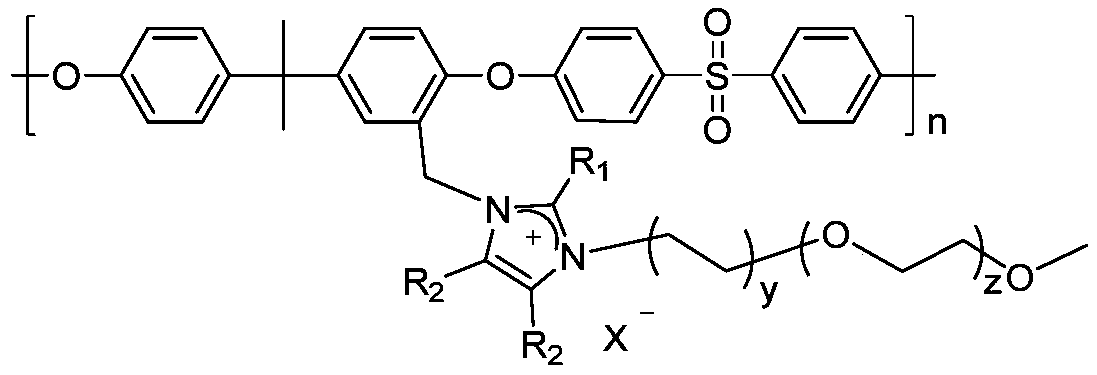
A kind of hydrophilic long side chain alkaline anion exchange membrane and preparation method thereof
A basic anion and exchange membrane technology, which is applied in electrochemical generators, fuel cells, electrical components, etc., can solve the problems of no membrane material reports, and achieve the effect of cheap and abundant raw materials, good application prospects, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Preparation of Im-2C-MEG: Under the protection of nitrogen, dissolve 20mmol sodium hydride in 40mL tetrahydrofuran, stir well, add 20mmol ethylene glycol monomethyl ether, react for 2 hours, add 60mmol 1,2-dibromoethyl to the above solution in alkanes, fully stirred, and reacted at 60°C for 8 hours; then the reaction solution was suction filtered, washed with water, and the aqueous phase was extracted three times with chloroform, the organic phases were combined, dried with anhydrous magnesium sulfate, concentrated by rotary evaporation, and refined by distillation under reduced pressure (46°C / 0.5mmHg) to obtain a colorless transparent liquid bromide. Under the protection of nitrogen, 10mmol 2-methylimidazole and 12mmol sodium hydride were fully reacted in 40mL tetrahydrofuran solution. After 1h, the bromide was added to the above reaction solution, and reacted at room temperature for 24h. Methane / methanol=20 / 1 was used as column chromatography developing solvent, and c...
Embodiment 2
[0039] Preparation of Im-2C-DEG: Under the protection of nitrogen, dissolve 20mmol sodium hydride in 40mL toluene, stir well, add 20mmol diethylene glycol monomethyl ether, react for 4 hours, add 80mmol 1,2-dibromo in ethane, fully stirred, and reacted at 80°C for 10 h; then the reaction solution was suction filtered, washed with water, and the aqueous phase was extracted 3 times with chloroform, and the organic phase was combined, dried with anhydrous magnesium sulfate, concentrated by rotary evaporation, and refined by distillation under reduced pressure (80 ℃ / 0.5mmHg), a colorless and transparent liquid bromide was obtained. Under the protection of nitrogen, 10mmol 2-methylimidazole and 12mmol sodium hydride were fully reacted in 40mL toluene solution. After 1h, the bromide was added to the above reaction solution, and reacted at room temperature for 24h. Methane / methanol=40 / 1 was used as column chromatography developing solvent, and column chromatography separation and pur...
Embodiment 3
[0043] Preparation of Im-2C-TEG: Under the protection of nitrogen, dissolve 20mmol sodium hydride in 40mL cyclohexane, stir well, add 20mmol triethylene glycol monomethyl ether, and react for 6 hours, add 100mmol 1,2-bis In bromoethane, fully stirred, reacted at 100°C for 16h; then the reaction solution was suction filtered, washed with water, and the aqueous phase was extracted 3 times with chloroform, the organic phase was combined, dried with anhydrous magnesium sulfate, concentrated by rotary evaporation, and refined by vacuum distillation ( 104°C / 0.5mmHg), a colorless transparent liquid bromide was obtained. Under the protection of nitrogen, 10mmol 2-methylimidazole and 12mmol sodium hydride were fully reacted in 40mL cyclohexane solution. After 1h, the bromide was added to the above reaction solution, and reacted at room temperature for 24h. After suction filtration and concentration, use Dichloromethane / methanol=60 / 1 was used as column chromatography developing solvent,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com