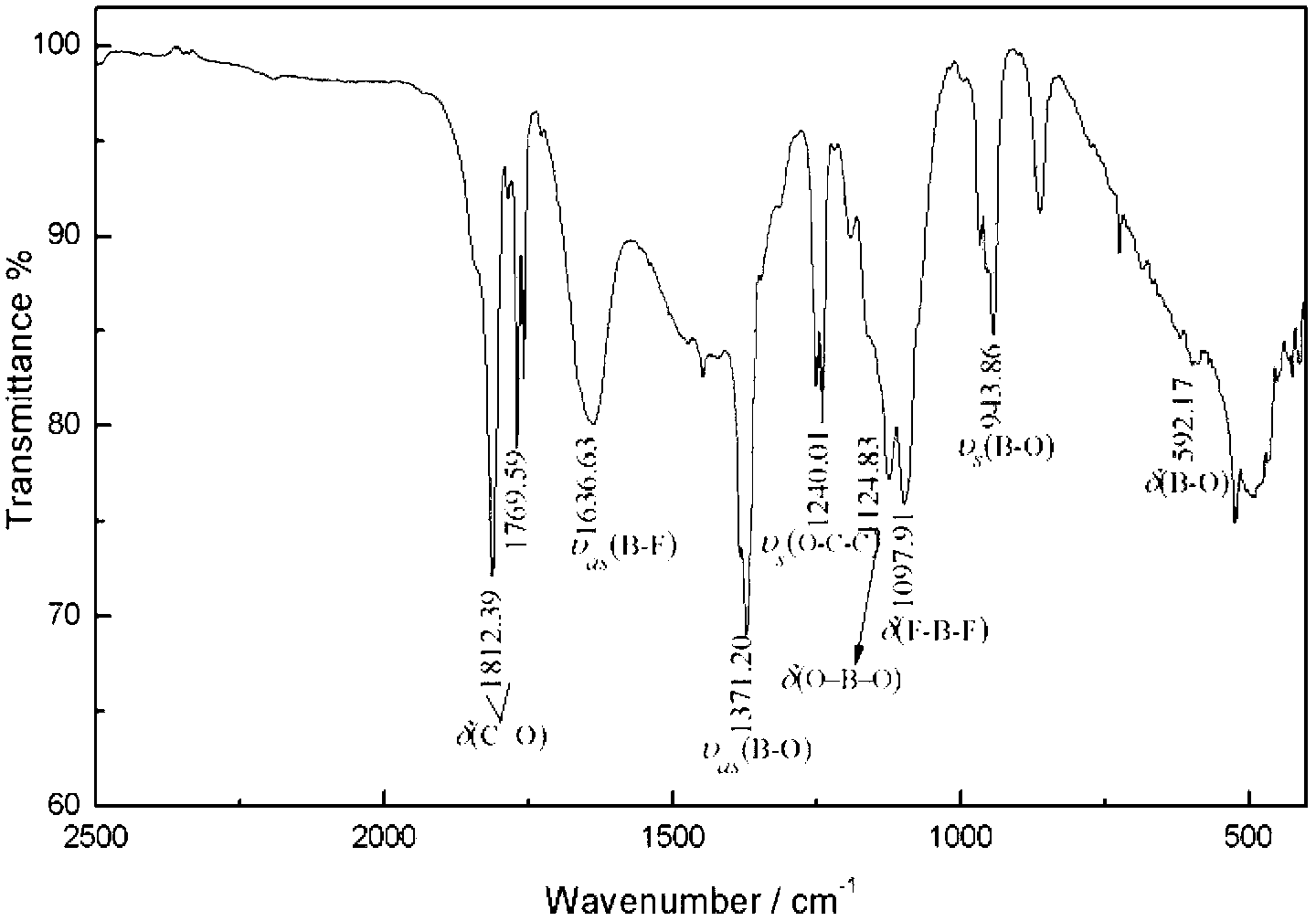
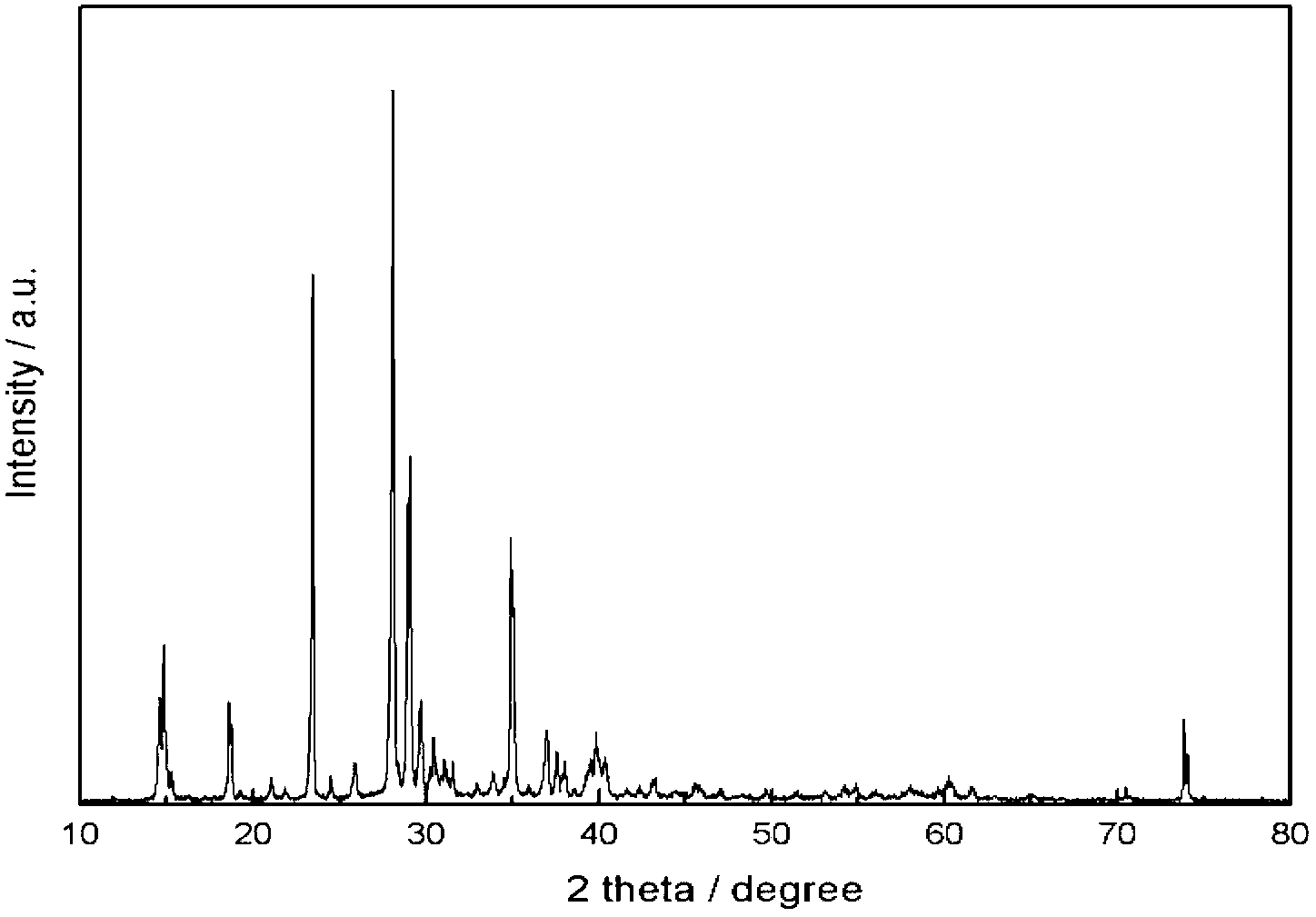
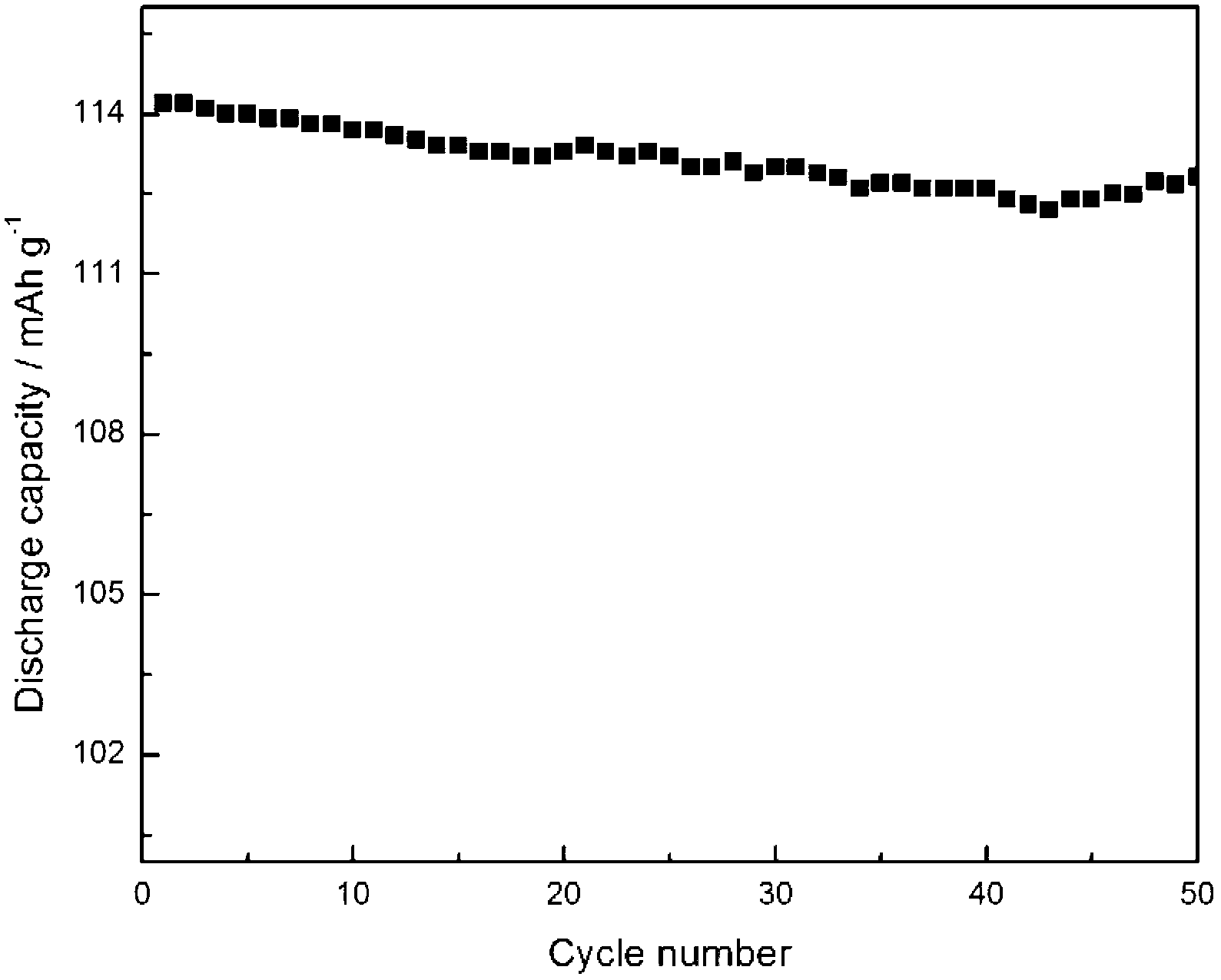
Method for preparation and purifying lithium difluoroborate
A technology of lithium difluorooxalate borate and purification method, which is applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problem of inability to form a stable SEI film, harsh synthesis conditions, ion Low conductivity and other problems, to achieve the effect of easy operation, simple synthesis method and shortened process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) First, dry 42.39g LiCl and 115g NaBF 4 Be raw material, add in the reactor that 100g DMC is housed as organic solvent and carry out magnetic stirring; Then in described reactor, add 2g tetraethylammonium bromide as catalyst; Heating while stirring, control reaction temperature is 50 ℃, After reacting for 6h, the insoluble matter was filtered to obtain LiBF 4 organic solution;
[0029] (2) With LiBF 4 The organic solution was used as raw material, and then anhydrous 100gH was added to the three-necked flask under an inert atmosphere 2 C 2 o 4 , while adding 2gAlCl 3 The catalyst was magnetically stirred at 90°C, and refluxed until no gas was produced, and the reaction was terminated;
[0030] (3) Filter and evaporate the filtrate with a rotary evaporator until white solid particles are just formed, then add non-polar solvent cyclohexane to it, the amount added is 2:1 according to the volume ratio of solvent to solution; Crystallize, filter, and dry under vacuu...
Embodiment 2
[0032] (1) First dry 42.39g LiCl and 120g NaBF 4 Be raw material, add in the reactor that 100g DMC is housed as organic solvent and carry out magnetic stirring; Then in described reactor, add 3g tetrapropylammonium bromide as catalyst; Heating while stirring, control reaction temperature is 70 ℃, After reacting for 4h, the insoluble matter was filtered to obtain LiBF 4 organic solution;
[0033] (2) With LiBF 4 The organic solution of the organic solution is used as a raw material, and then anhydrous 105gH is added to the three-necked flask under an inert atmosphere 2 C 2 o 4 , while adding 2gAlCl 3 Catalyst, magnetic stirring at 100°C, reflux until no gas is generated, and the reaction is terminated;
[0034] (3) Filtrate and evaporate the filtrate with a rotary evaporator until white solid particles are just formed, then add non-polar solvent toluene to it, the amount added is 5:1 according to the volume ratio of solvent to solution; recrystallize at low temperature, ...
Embodiment 3
[0036] (1) First dry 42.39g LiCl and 115g NaBF4 As a raw material, add 95g DMC as an organic solvent to a reactor for magnetic stirring; then add 2.5g 18-crown-6 as a catalyst to the reactor; heat while stirring, and control the reaction temperature to 80°C , after reacting for 10h, filter the insoluble matter to obtain LiBF 4 organic solution;
[0037] (2) With LiBF 4 The organic solution was used as raw material, and then anhydrous 100gH was added to the three-necked flask under an inert atmosphere 2 C 2 o 4 , while adding catalyst 2g SiCl 4 , stirred magnetically at 80°C until no gas was generated, and the reaction was terminated;
[0038] (3) Filter, and evaporate the filtrate with a rotary evaporator until the white solid particles are just formed, then add the non-polar solvent cyclohexane to it, the amount added is 4:1 according to the volume ratio of the solvent to the solution; Crystallize, filter, and dry under vacuum at 60°C to obtain a lithium difluorooxalate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com