Rubrene analogue, as well as preparation method and application thereof
A reaction and compound technology, applied in the field of rubrene analogues and their preparation, achieves the effects of good repeatability, high universality, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
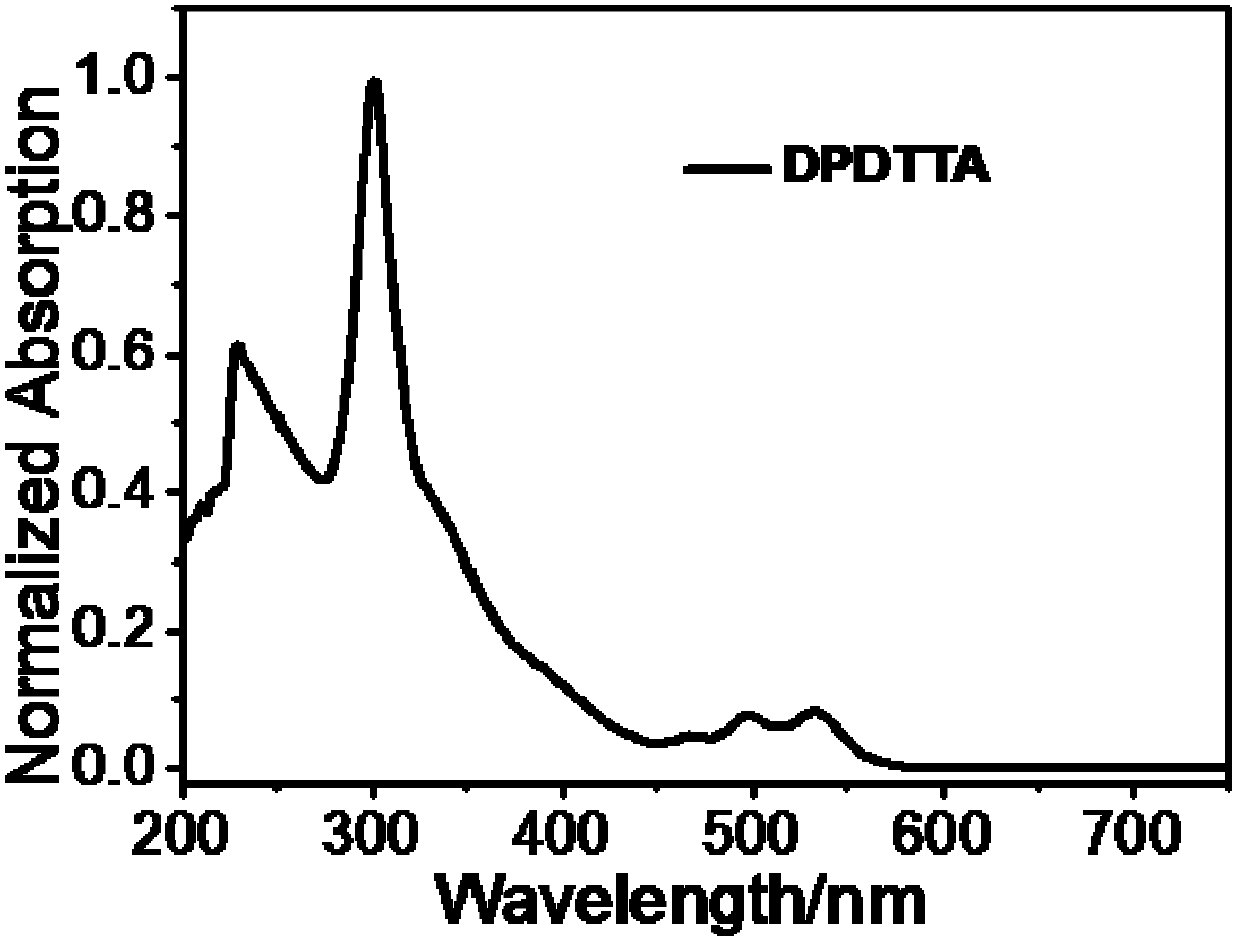
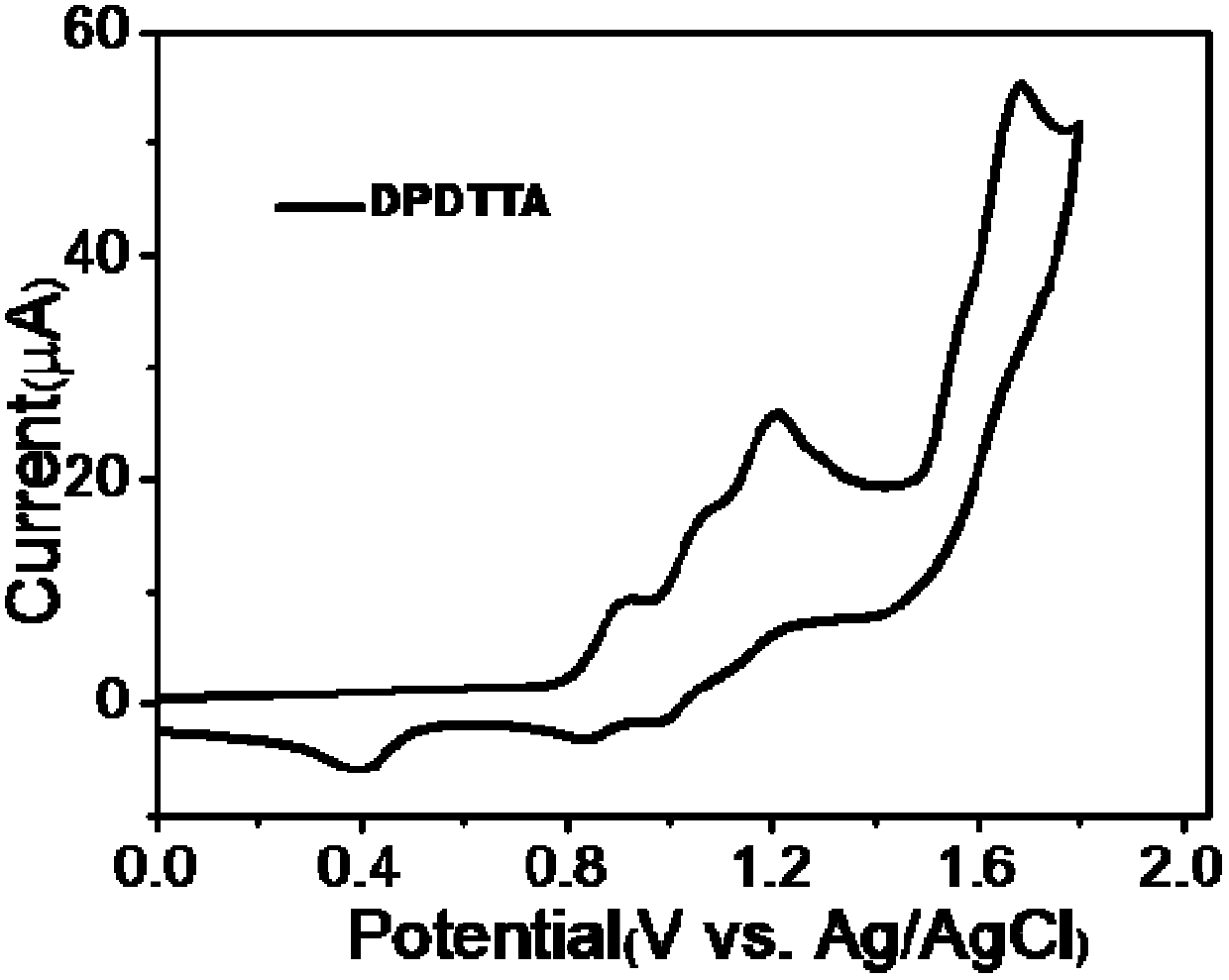
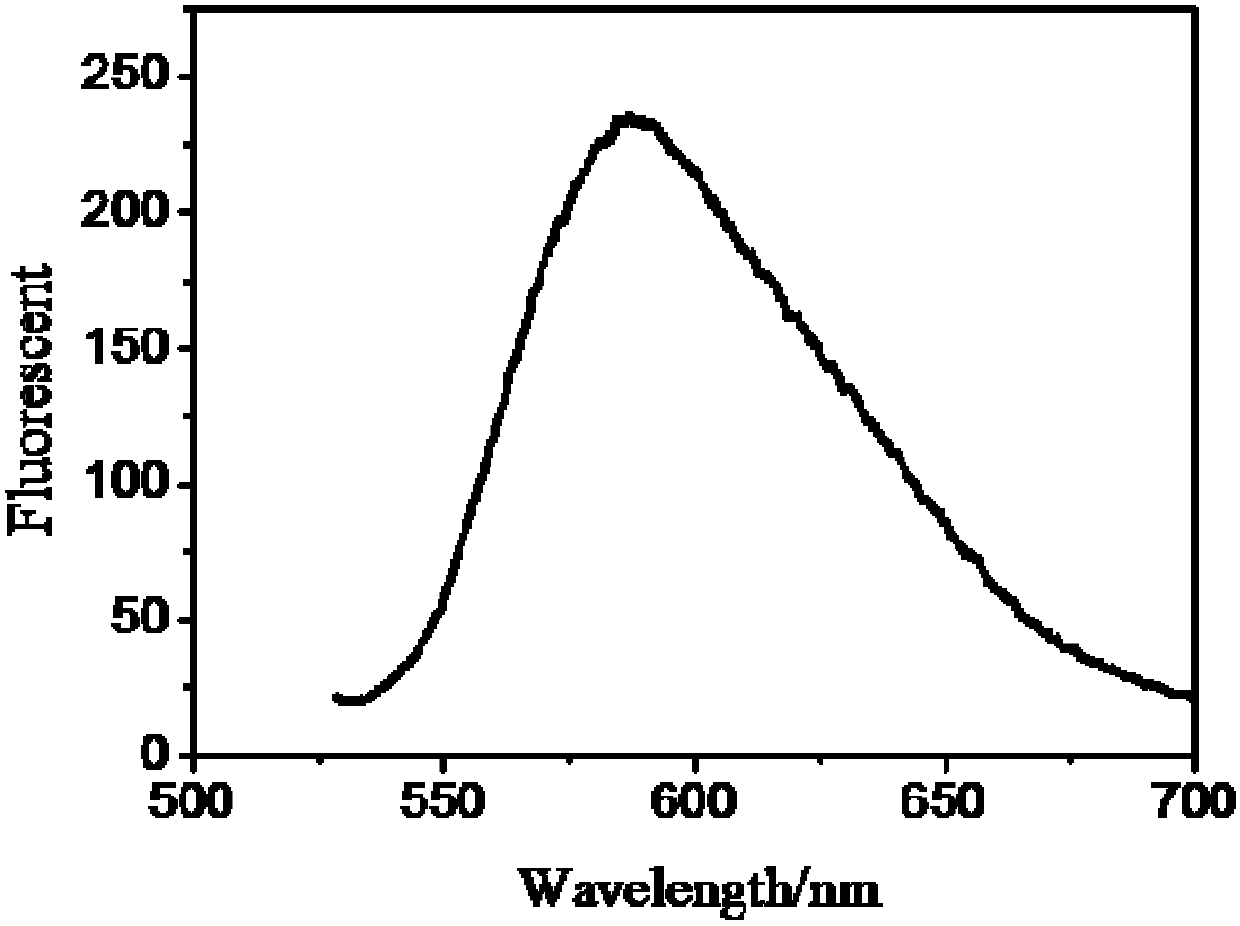
[0037] Example 1. Preparation of 5,11-diphenyl-6,12-dithienyltetracene (DPDTTA) represented by formula I
[0038] In a 50mL two-necked flask, add 1,1-diphenyl-3-(2-thienyl)propynyl alcohol (1.45g, 5mmol) represented by formula II, add 30ml of dry solvent toluene, and evacuate with argon. Slightly heat and stir until it is completely dissolved, place the flask in an ice-water bath, add the single electron transfer reagent N,N-diisopropylethylamine (1.75mL, 8.5mmol), and slowly add the electrophile benzenesulfonate dropwise after 5min Acyl chloride (0.85mL, 7.5mmol), reacted at 0°C-5°C for 20min, heated to 110°C for 10h. Cool down to room temperature and finish the reaction. Wash the extraction with ethyl acetate (50mL) and 2M hydrochloric acid solution, dry the organic liquid with anhydrous magnesium sulfate, and purify it with a silica gel column (the eluent is petroleum ether to obtain 273 mg of a red solid ( Yield: 20%).
[0039] The structural characterization data of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hole mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com