Method for reducing and removing metal impurities from ammonium molybdate solution by using ion exchange resin
A technology for ion exchange resins and metal impurities, applied in chemical instruments and methods, molybdenum compounds, inorganic chemistry, etc., can solve the problems of resin ion exchange performance adverse effects, loss of adsorption, etc., to achieve easy desorption regeneration, reduce impurities removal Effects of cost reduction and reduction of running time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
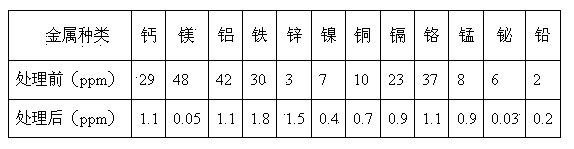
[0030] Amberlite IRC-84 type weakly acidic acrylic cation exchange resin produced in the United States, 732 type strongly acidic styrene type cation exchange resin produced by Zhejiang Zhengguang Resin Company, and D168 type weakly acidic phenolic cation exchange resin produced by Shanghai Jinyang Resin Company were selected. Add three times the resin volume and 5% ammonia water to the three resins for soaking and rinsing. The treatment time is 1 hour. Resin transformation treatment.
[0031] Take a certain amount of ammonium molybdate purification solution, add nitric acid with a mass concentration of 50% dropwise under stirring conditions, and adjust its pH value to close to 5.
[0032] The transformed Amberlite IRC-84, 732 and D168 cation exchange resins were loaded into the exchange columns in turn, the ratio of the filling height to the diameter of the exchange column was 10:1, and then the three exchange columns were connected in series in sequence, and the molybdic acid...
Embodiment 2
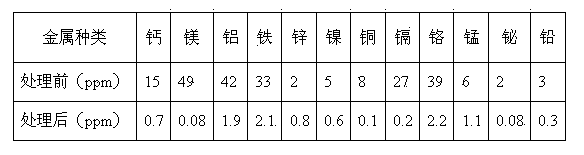
[0039] Amberlite IRC-72 weakly acidic acrylic cation exchange resin produced in the United States, Diaion SK-IA strong acidic styrene cation exchange resin produced in Japan, and D126 weakly acidic phenolic cation exchange resin produced by Shanghai Jinkai Resin Company were selected. , respectively add 5 times the volume of the resin and 10% ammonia water to the three resins for soaking and rinsing. The treatment time is 3 hours. Realize the transformation process of resin.
[0040] Take a certain amount of ammonium molybdate purification solution, add nitric acid with a mass concentration of 60% dropwise under stirring conditions, and adjust its pH value to close to 7.
[0041] The transformation-treated Amberlite IRC-72, Diaion SK-IA and D126 cation exchange resins were loaded into the exchange columns in turn, and the ratio of the filling height to the diameter of the exchange column was 20:1, and then the three exchange columns were connected in series. Add the ammonium ...
Embodiment 3
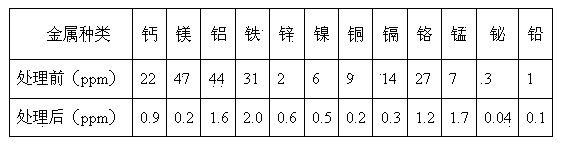
[0048] Duolite C-464 weakly acidic acrylic cation exchange resin produced in France, Amberlite IR-120 strongly acidic styrene cation exchange resin produced in the United States, and D155 weakly acidic phenolic cation exchange resin produced by Zibo Dongda Resin Company were selected. , respectively add 4 times the volume of the resin and ammonia water with a mass concentration of 7% to the three resins for soaking and rinsing. The treatment time is 2 hours. Realize the transformation process of resin.
[0049] Take a certain amount of ammonium molybdate purification solution, add nitric acid with a mass concentration of 55% dropwise under stirring conditions, and adjust its pH value to close to 6.
[0050] The transformation-treated Duolite C-464, Amberlite IR-120 and D155 cation exchange resins were loaded into the exchange columns in turn, and the ratio of the filling height to the diameter of the exchange column was 15:1, and then the three exchange columns were connected ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com