Gimeracil crystal form and preparation method thereof
A technology for gimeracil and crystal, which is applied in the field of gimeracil C-type crystal and its preparation, can solve the problems affecting drug efficacy and stability, melting point, solubility, dissolution, bioequivalence difference, etc., to meet the needs of the pharmaceutical industry , The effect of stable properties and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation of embodiment 1 Gimeracil type C crystals
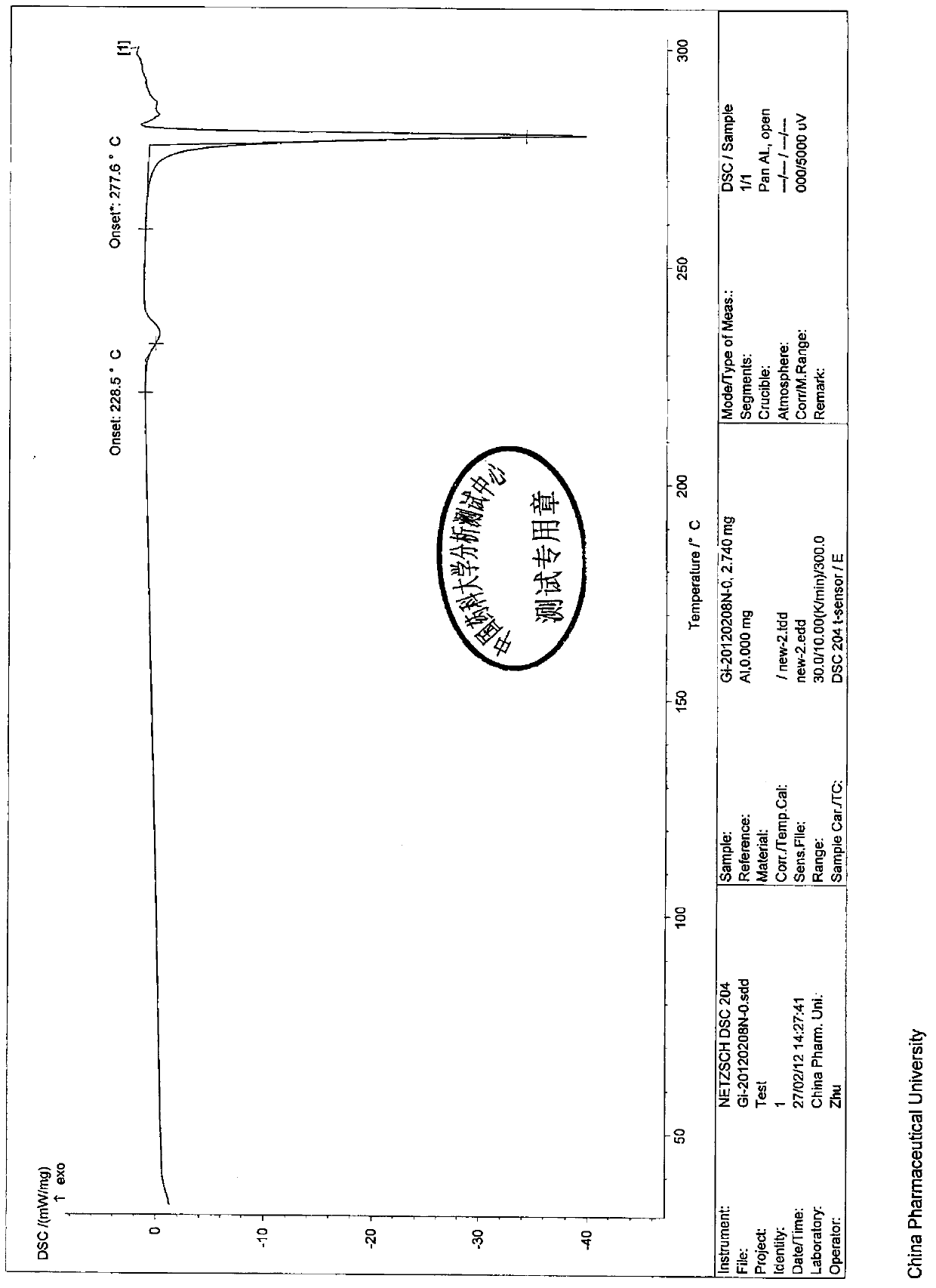
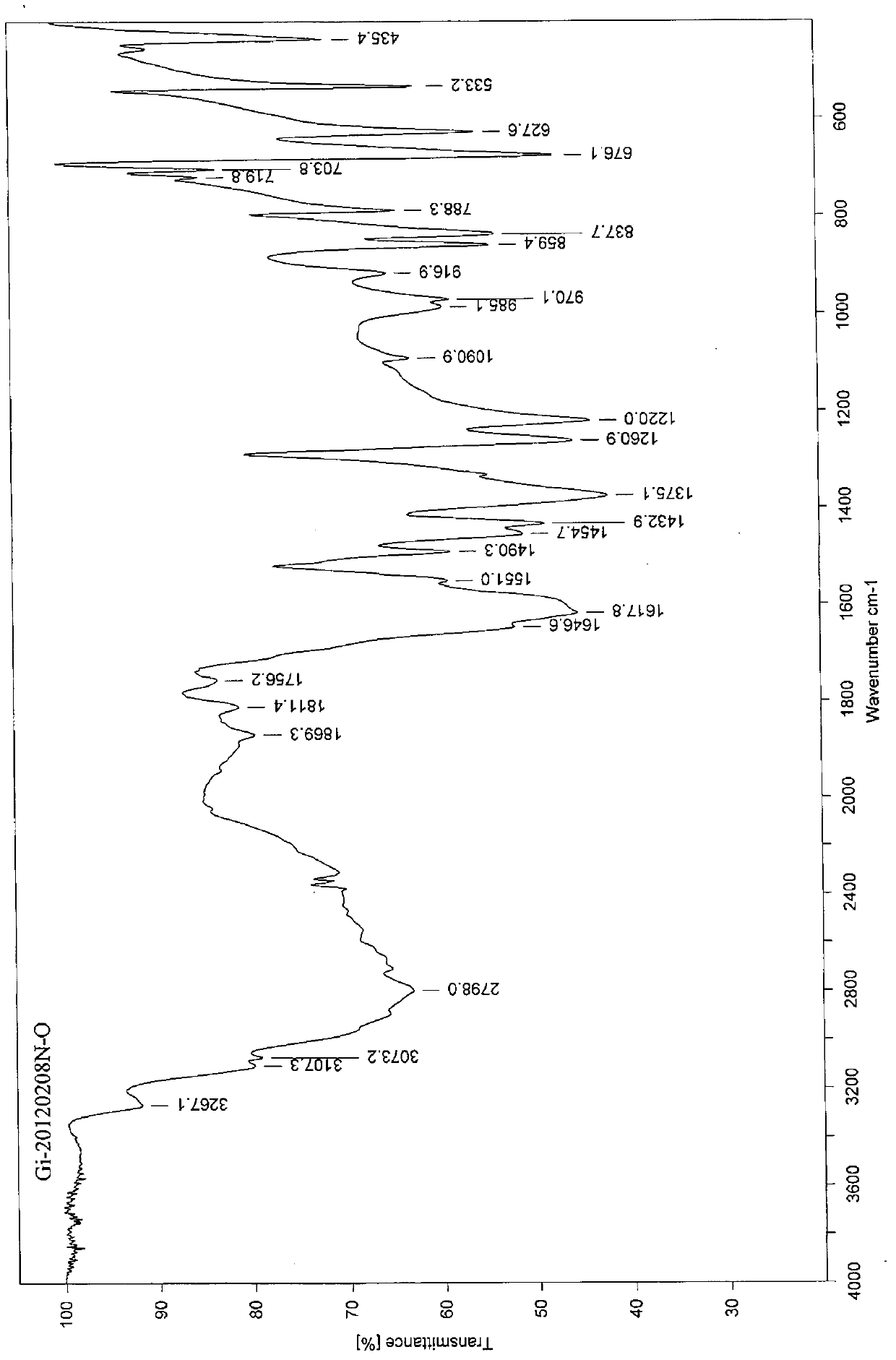
[0024] 100g of commercially available gimeracil was heated and dissolved in 5L of a mixed solvent composed of ethanol, acetonitrile and water, and the volume percentage of ethanol: acetonitrile: water in the mixed solvent was 15%: 10%: 75%, stirred at 70°C to dissolve it , suction filtration while hot, cooling and crystallization of the filtrate for 16-24 hours, suction filtration, and drying to obtain 89 g of white powdery solid, with a yield of 89%, melting point: 277-278°C, and a purity of 99.92% as determined by HPLC.
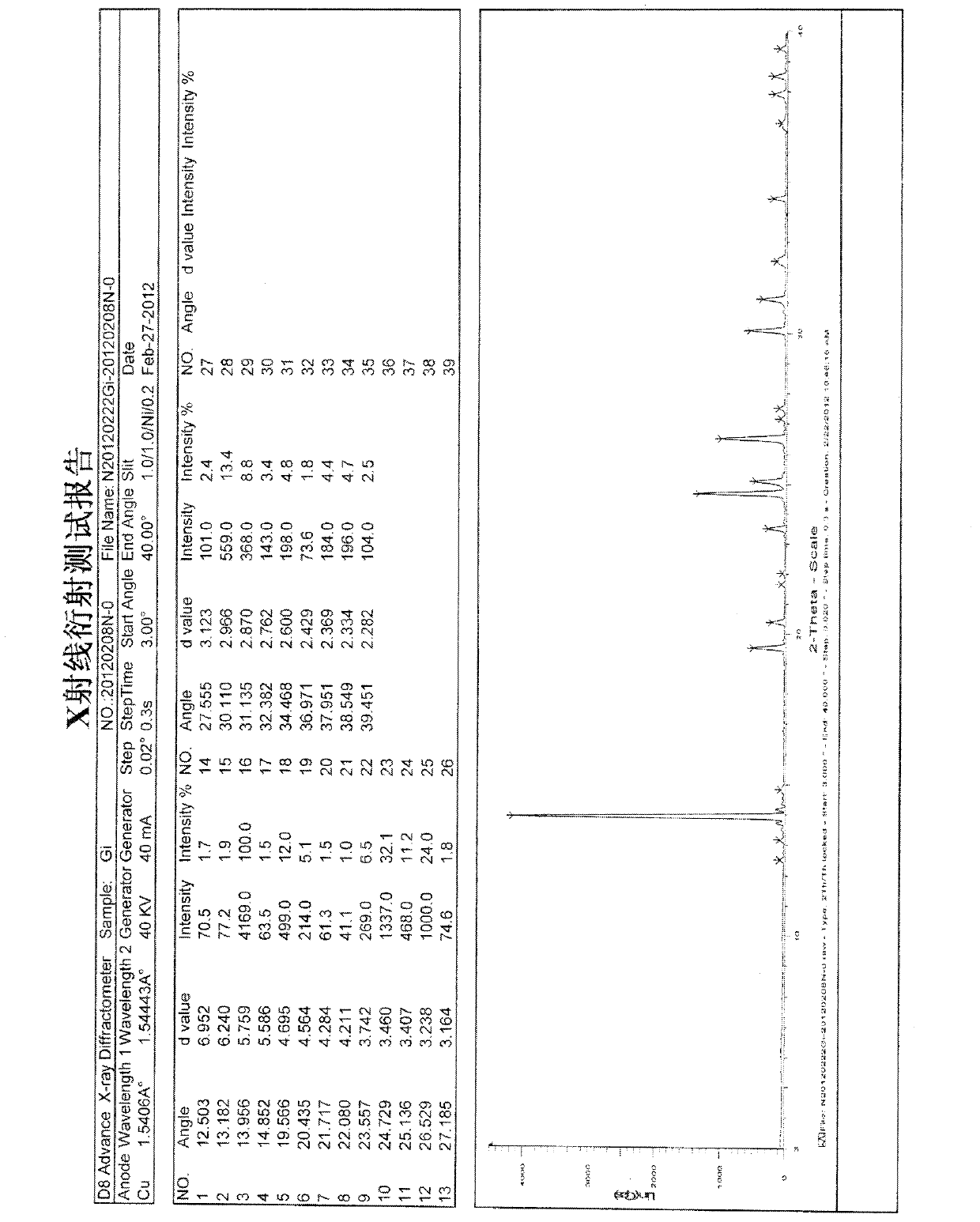
[0025] Embodiment 2 Apparatus and test conditions used for identification of gimeracil C-type crystal crystal form
[0026] (i) Powder X-ray Diffraction:
[0027] Instrument: Bruker D8Advance X-ray diffractometer
[0028] Test condition: 40kv 40mA Slit: 1.0 / 1.0 / Ni / 0.2 Step size: 0.02°
[0029] Target type: Cu
[0030] (ii) Differential scanning thermal analysis:
[0031] Instrument: NET...
Embodiment 4
[0039] The preparation of embodiment 4 pharmaceutical composition
[0040] Put 0.58kg of gimeracilidine type C crystal prepared in Example 1, 2kg of tegafur, 1.96kg of oteracil potassium, 8.3kg of lactose, and 0.26kg of sodium lauryl sulfate into the granulator, and start stirring at 1000rpm Mix for 5 minutes, then slowly add 5 kg of 3% hydroxypropyl cellulose solution, and continue stirring for 1 minute after the addition is complete to obtain wet granules. The wet granules were dried at 80°C until the water content was 3% to obtain dry granules. The obtained dry granules are mixed with 0.065kg of magnesium stearate, and further made into capsules by conventional methods in the formulation field.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com