Preparation method of thienopyridine ketone compounds
A compound, pyridone technology, applied in the field of preparation of thienopyridone compounds, can solve the problems of limited application range and many synthesis steps, and achieve the effects of wide application range, simple synthesis method and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
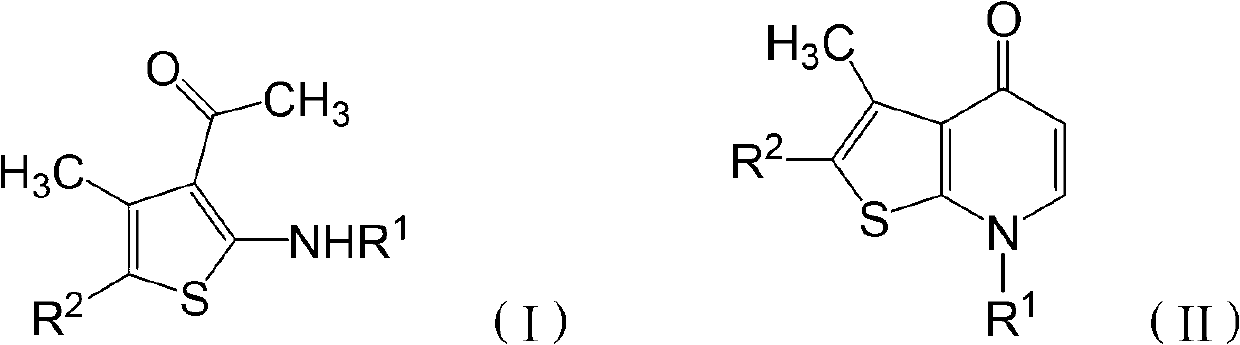
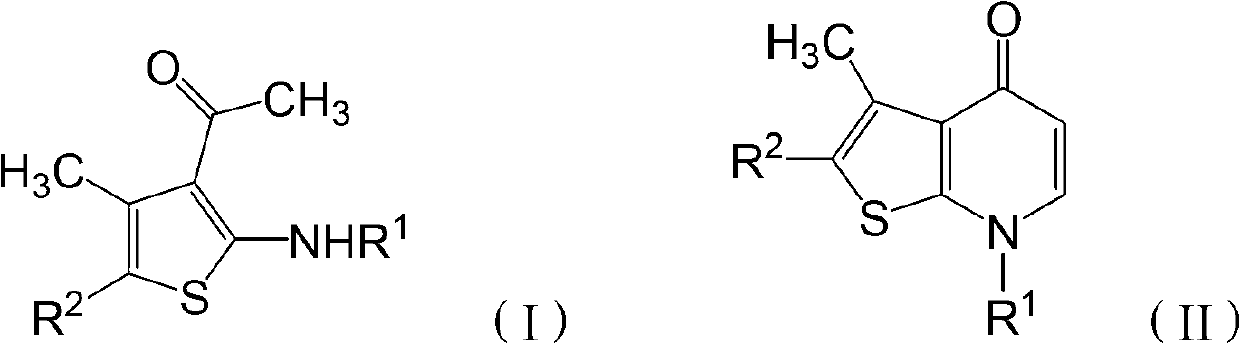
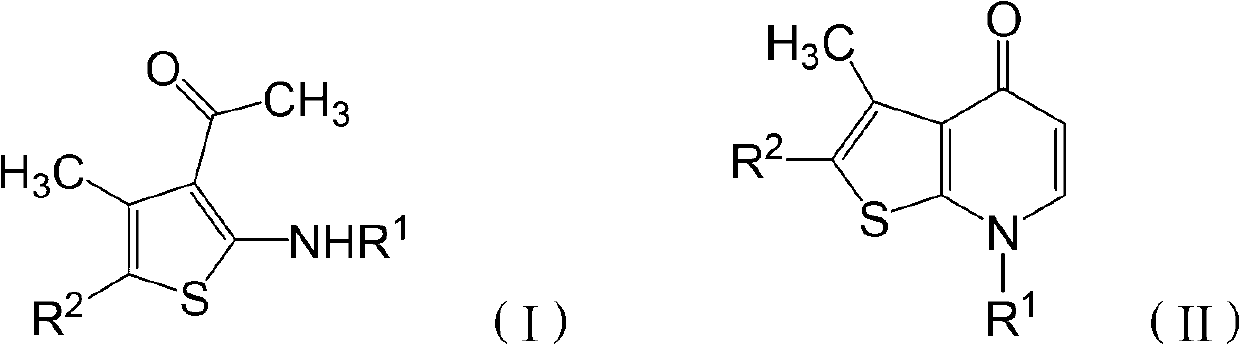
[0029] The invention provides a preparation method of thienopyridone compounds, which comprises the following steps: distilling 2-amino-3-acetylthiophene compounds with the structure of formula (I) and N,N-dimethylformamide Methyl acetal reaction to obtain thienopyridone compounds with the structure of formula (II).
[0030]
[0031] Among them, R 1 -H, -A, -COA, -CONA 2 , -COAr, -SO 2 A. -SO 2 Ar, -Ar, or -Het; R 2 -COA, -COAr, -COOA, -Ar or -Het; said A is C1~C30 chain alkyl, C1~C30 substituted chain alkyl or C3~C7 cycloalkyl; said Het is 1 Monocyclic unsaturated heterocycles with ~4 heteroatoms, bicyclic unsaturated heterocycles with 1 to 4 heteroatoms, substituted monocyclic unsaturated heterocycles with 1 to 4 heteroatoms or 1 to 4 heteroatoms A substituted bicyclic unsaturated heterocycle, the heteroatom is one or more of N, O and S atoms. When Het is a bicyclic unsaturated heterocyclic ring or a substituted bicyclic unsaturated heterocyclic ring, the heteroatom...
Embodiment 1
[0055] At room temperature, mix and stir 10mmol acetylacetone, 20mmol anhydrous potassium carbonate and 30ml N,N-dimethylformamide (DMF), slowly add 10mmol phenylisothiocyanate, react for 2h, add 10mmolβ-bromobenzene Ethanone, after continuing to react for 2 hours, pour the reaction solution into saturated saline, extract three times with 20ml of dichloromethane, combine the organic phases, wash with 20ml of water three times, add 5g of anhydrous sodium sulfate to dry, filter, remove the organic solvent, pass through silica gel Column chromatography separated to obtain 2-anilino-3-acetyl-4-methyl-5-benzoylthiophene.
[0056] The preparation method of the 2-amino-3-acetylthiophene compound used in the following examples is the same as above, the difference is that the raw material isothiocyanate with the structure of formula (III) and β-bromo with the structure of formula (IV) Compounds are chosen differently.
[0057] 2-(4-Methoxyanilino)-3-acetyl-4-methyl-5-(4-nitrophenyl)th...
Embodiment 2
[0070] Add 2mmol of 2-anilino-3-acetyl-4-methyl-5-benzoylthiophene obtained in Example 1, 4mmol DMFDMA and 20ml DMF to a 50ml round bottom flask equipped with a reflux condenser and a stirrer , heated to 120°C under stirring conditions, and reacted for 2 hours, poured the reaction liquid into 100ml saturated saline, extracted twice with 30ml dichloromethane, combined the organic phases, washed twice with 30ml water, added 5g anhydrous sulfuric acid Sodium drying, filtration, removal of organic solvent, separation by silica gel column chromatography to obtain 2-benzoyl-3-methyl-7-phenylthieno[2,3-b]pyridin-4[7H]ketone, yield 90%. The reaction formula is as follows:
[0071]
[0072] The 2-benzoyl-3-methyl-7-phenylthieno[2,3-b]pyridin-4[7H]ketone obtained in Example 2 is analyzed by nuclear magnetic resonance to obtain its hydrogen spectrum and carbon Spectrum, the result is as follows:
[0073] 1 H NMR (300MHz, CDCl 3 ): δ2.66(s,3H),6.39(d,J=7.8Hz,1H),7.44–7.60(m,9H),7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com