A kind of synthetic method of porphyrin
A synthesis method and technology of porphyrin, which are applied in the field of synthesis and purification of substituted porphyrin, can solve the problems of troublesome operation, low yield, difficult separation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
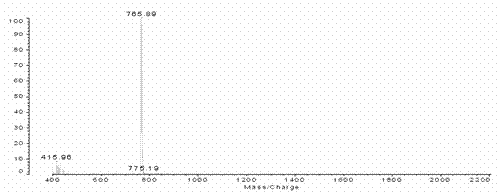
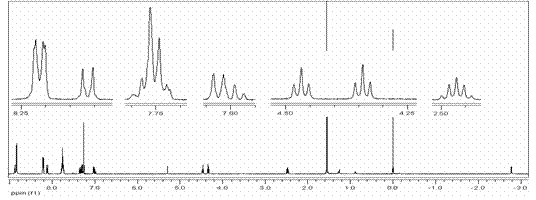
[0029] Add 1.03g (4mmol) 2-(3-phenoxy) propoxybenzaldehyde, 0.89g (4mmol) dipyrrolidine and 600ml solvent chloroform in the 1000ml three-necked flask, stir at room temperature for 15 minutes under nitrogen protection , then 0.03 mL (0.25 mmol) of BF 3 ·Et 2 O dissolved in 10 mL CHCl 3 Slowly added dropwise to the reactor. After stirring for 24 hours, add 0.68 g (3 mmol) DDQ and stir for 30 hours. The reaction solution is decompressed and spin-dried to dry the solvent, and then the black viscous oily liquid is dissolved with a small amount of dichloromethane and passed through a silica gel column (silica gel: 200-300 mesh, column: 2.5cm in diameter, 40cm in length, 35cm in packing height), CH 2 Cl 2 As a developing agent, the first dark purple band is the mixed crude product. According to the actual situation, the column can be passed again under the same conditions. Then the mixture product was spin-dried, dissolved in 2mL of dichloromethane, and then passed through sili...
Embodiment 2
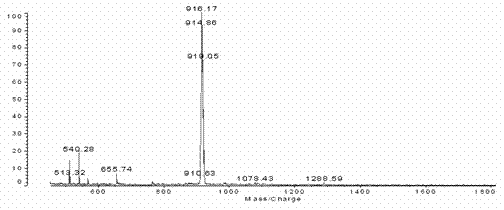
[0037] Add 1.03g (4mmol) 4-(3-phenoxy) propoxybenzaldehyde, 0.89g (4mmol) dipyrrolidine and 600ml solvent chloroform in the 1000ml three-necked flask, stir at room temperature for 15 minutes under nitrogen protection , then 0.03 mL (0.25 mmol) of BF 3 ·Et 2 O dissolved in 10 mL CHCl 3 Slowly added dropwise to the reactor. After stirring for 24 hours, add 0.68 g (3 mmol) DDQ and stir for 30 hours. The reaction solution is decompressed and spin-dried to dry the solvent, and then the black viscous oily liquid is dissolved with a small amount of dichloromethane and passed through a silica gel column (silica gel: 200-300 mesh, column: 2.5cm in diameter, 40cm in length, 35cm in packing height), CH 2 Cl 2 As a developing agent, the first dark purple band is the mixed crude product. According to the actual situation, the column can be passed again under the same conditions. Then the mixture product was spin-dried, dissolved in 2mL of dichloromethane, and then passed through sili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com