Non-natural chiral amino acid and biological catalysis desymmetrisation preparation method thereof
A chiral and compound technology, applied in the field of non-natural chiral amino acid and its biocatalytic desymmetry preparation, can solve the problems of poor selectivity and harsh chemical transformation requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] References for the preparation of malononitrile compounds with two different substituents: Enrique Diez-Barra; Antonio de la Hoz; Andres Moreno and Prado Sanchez-Verdu.J.CHEM.SOC.PERKIN TRANS.1991, 1, 2589. The preparation method of the compound not in the literature is the same as the preparation method of allylmethylmalononitrile.
[0064] The preparation method of allylmethylmalononitrile: dissolve NaH (5mmol) in the mixed solvent DMF (2mL) and THF (20mL), add the substrate malononitrile (10mmol), stir at 0°C for 1 hour, then add Allyl bromide (5mmol), continue stirring for 4 hours and add H 2 O (20mL), extracted three times with ethyl acetate (25), MgSO 4 Drying, removal of solvent, separation by column chromatography to obtain allyl malononitrile. Dissolve allyl malononitrile (2mmol) in acetone (10mL), add K 2 CO 3 (4mmol) and CH 3 I (10 mmol), stirred at room temperature for 3 hours, filtered, removed the solvent, and separated by column chromatography to obt...
Embodiment 1
[0067] Embodiment 1: preparation compound 1
[0068]
[0069] Specific implementation method:
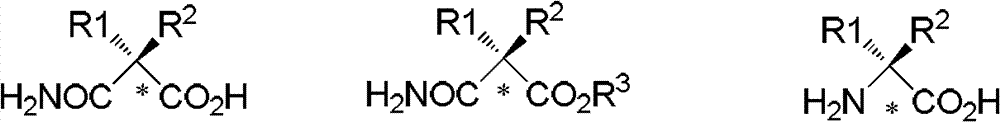
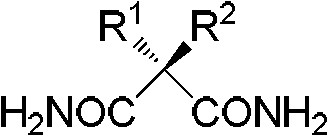
[0070] 1) Take 2 grams of wet weight of Rhodococcus erythropolis AJ270 cells (Institute of Chemistry, Chinese Academy of Sciences), thaw at 30°C for 30 minutes, and use a buffer solution of dipotassium hydrogen phosphate and potassium dihydrogen phosphate (0.1M, pH 7.0, 50ml ) wash the thalline into the Erlenmeyer flat-bottomed flask with threaded mouth, disperse and shake well and put it into a shaker for activation at 30°C for 30 minutes, then add 1 mmol (156 mg) of the formula IV-1 at one time The compound was placed in a shaker at 30° C. and 200 rpm to carry out catalytic hydrolysis reaction. Whole reaction TLC monitors, stops reaction after reacting 0.5 hour, and gained reaction solution removes thalline by suction filtration of one layer of diatomaceous earth, washes filter residue three times with 20 milliliters of water successively, obtains the compound shown in formula...
Embodiment 2
[0074] Embodiment 2: preparation compound 2
[0075]
[0076] 1) Take two grams of wet weight of Rhodococcus erythropolis AJ270 cells (Institute of Chemistry, Chinese Academy of Sciences), thaw at 30°C for 30 minutes, and use a buffer solution of dipotassium hydrogen phosphate and potassium dihydrogen phosphate (0.1M, pH 7.0, 50ml ) wash the thalline into 150 milliliters of Erlenmeyer flat-bottomed flasks with threaded openings, disperse and shake well, and then put into a shaker to activate at 30°C for 30 minutes, then add 1 mmol (206 mg) of formula IV-2 at one time The compounds shown were placed in a shaker at 30° C. and 200 rpm for catalytic hydrolysis. Whole reaction TLC monitors, stops reaction after reacting 8h, and gained reaction solution is removed thalline through one layer of diatomaceous earth suction filtration, washes filter residue three times with 20 milliliters of water successively, obtains the compound shown in formula I-1 provided by the invention (R 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com