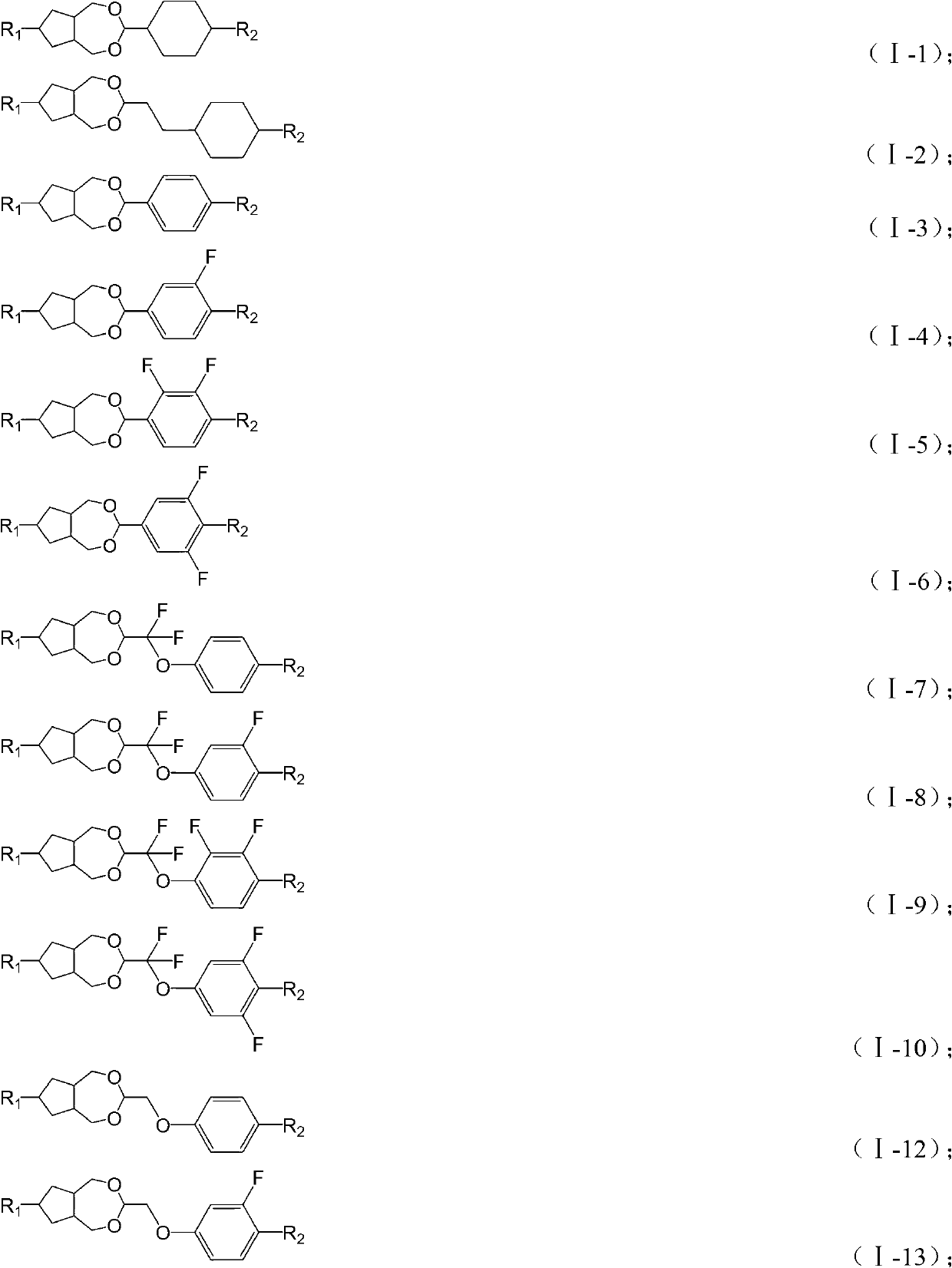
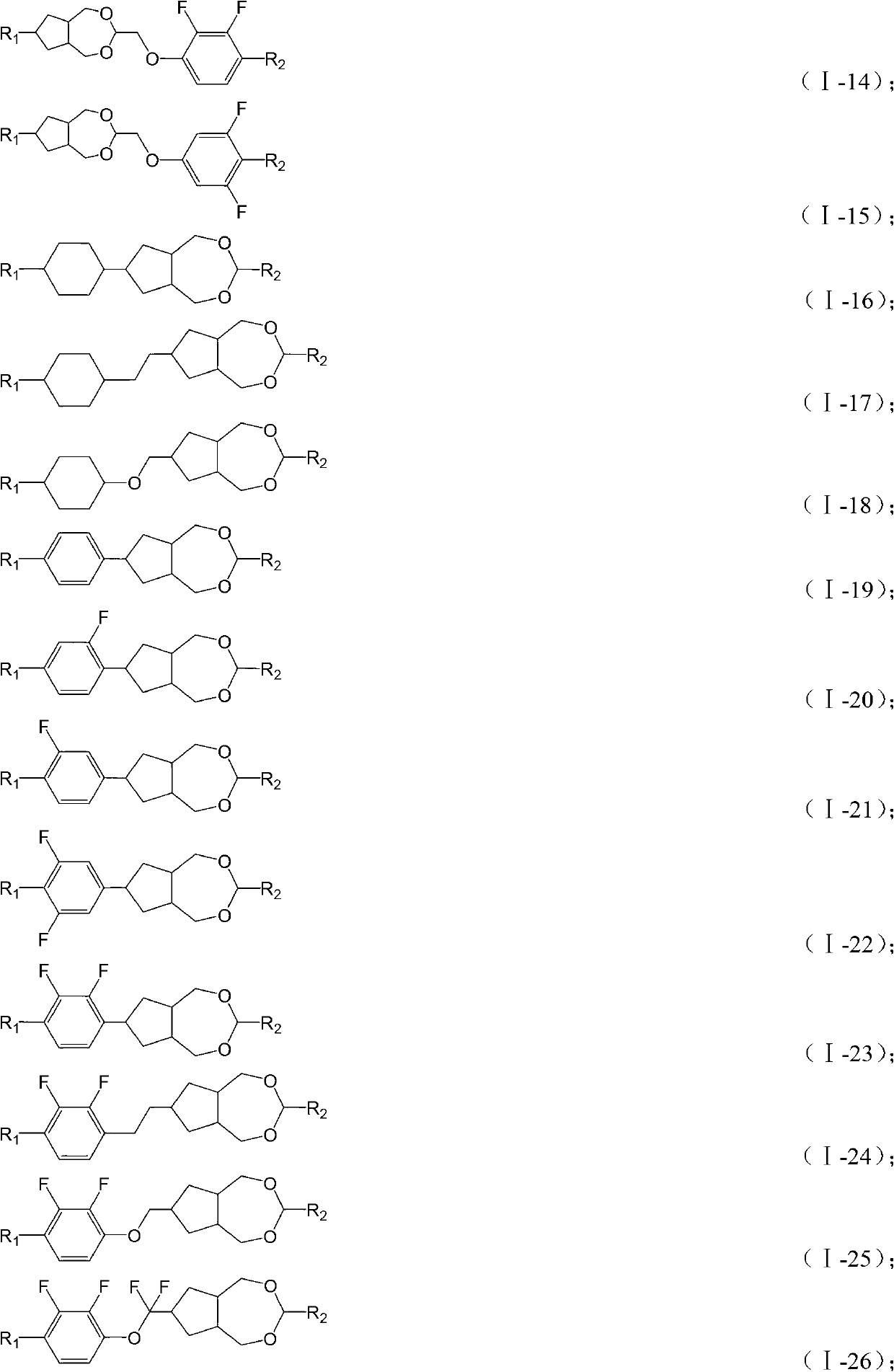
Novel liquid crystal compound containing dioxa saturated azulenes and composition thereof
A technology of compounds and heteroatoms, applied in the field of liquid crystal compounds and compositions comprising said liquid crystal compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The synthetic route for preparing compound 3(Da)CUF is as follows:
[0078]
[0079] Its specific preparation process is as follows:
[0080] 1. Synthesis of 3(Da)CUF-02
[0081] 3(Da)CUF-02
[0082] Add 3g of dimethyl succinate, 120mL of anhydrous tetrahydrofuran, 5ml of hexamethylphosphinamide into a 250mL three-necked flask, lower the temperature to -75°C under the protection of argon, and add dropwise 20mL of 1mol / L lithium diisopropylamide solution , after the drop is completed, stir at -80°C~-75°C for 45min, then add dropwise 9g of 2-propyl-1,3-dibromopropane (commercially available intermediate) in 30mL of anhydrous tetrahydrofuran solution at -80°C~-75°C , the dropwise addition was completed, stirred at -80°C~-75°C for 2h, then naturally warmed to room temperature and reacted for 20h; the reaction solution was poured into 50mL saturated aqueous ammonium chloride solution, transferred to a separatory funnel for liquid separation, and the water layer was wa...
Embodiment 2
[0091] The synthetic route for preparing compound 3C(Da)PUF is as follows:
[0092]
[0093] Its specific preparation process is as follows:
[0094] Among them, 2-(4-trans-propylcyclohexyl)-1,3-propanediol can be synthesized by methods known in the prior art (for the synthesis method, please refer to document CN101050157).
[0095]
[0096] 1. Synthesis of 3C(Da)PUF-03
[0097] 3C(Da)PUF-03
[0098] Add 15g of 2-(4-trans-propylcyclohexyl)-1,3-propanediol and 100ml of chloroform into a 250mL three-necked flask, install a drying, reflux and tail gas absorption device, and add 20g of tribromide dropwise at 0-5°C under stirring Phosphate 50ml chloroform solution, after dripping, stir at room temperature for 30min, then reflux reaction for 6h, TLC monitors no raw material, stop heating, naturally cool to room temperature, slowly add the reaction solution into 200ml saturated sodium phosphate ice solution , and then adjust the pH to 14 with 1mol / L sodium hydroxide soluti...
Embodiment 3
[0110] The synthetic route for preparing compound 3 (Da) PUF is as follows:
[0111]
[0112] Its specific preparation process is as follows:
[0113] 3(Da)CUF-01 Synthesis of Reference Example 1
[0114]Add 0.73g 3(Da)CUF-01, 1.0g 4-(3,4,5-trifluorophenyl)-benzaldehyde, 0.2g p-toluenesulfonic acid monohydrate, 100ml dichloromethane into a 250mL three-necked flask, Heated in a water bath to reflux to separate water, reacted for 6 hours, GC monitored no 4-(3,4,5-trifluorophenyl)-benzaldehyde, cooled naturally to room temperature; poured the reaction solution into 20mL saturated aqueous sodium bicarbonate solution, transferred to Separation in a separatory funnel, the aqueous layer was extracted twice with 10 mL of dichloromethane, the organic layer was combined and washed twice with saturated aqueous sodium bicarbonate solution, the organic layer was dried with 10 g of anhydrous sodium sulfate, and the solvent was evaporated with a rotary evaporator , to obtain 1.7 g of br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com