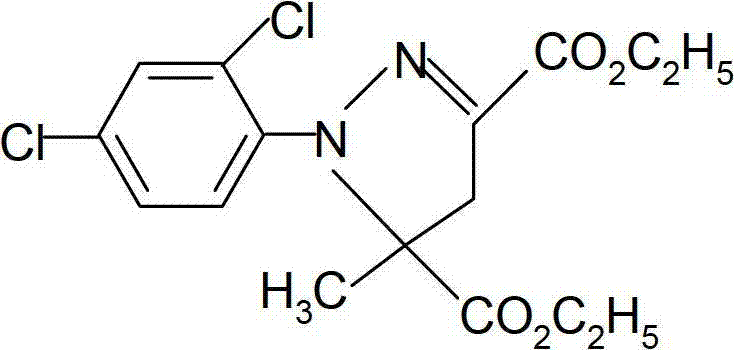
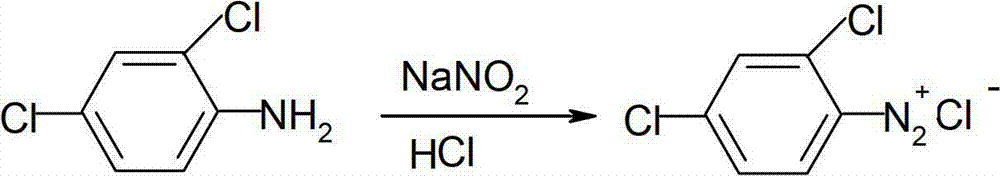
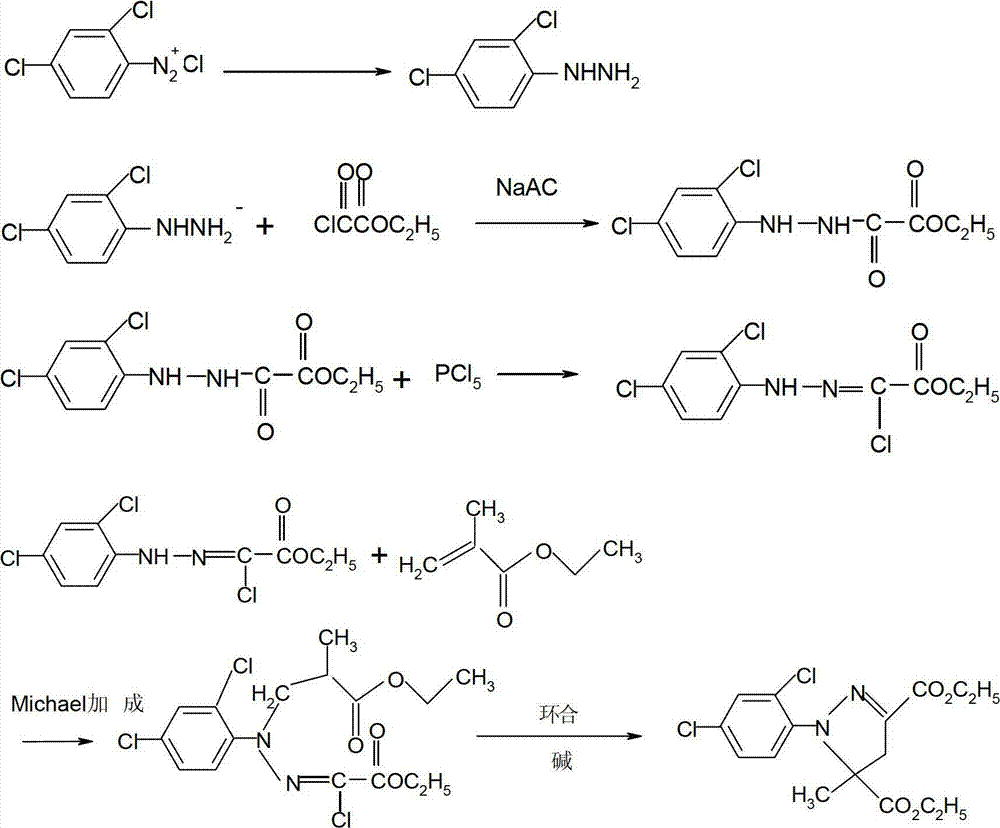
Catalytic synthesis method of mefenpyr-diethyl
A technology of pyrazole and cyclization reaction, applied in the direction of organic chemistry and the like, can solve the problems of complex process and low yield, and achieve the effects of simple process, high product quality and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the synthesis of ethyl chloroacetoacetate
[0025] Add 1 mol of ethyl acetoacetate into the reaction flask, start stirring, and lower the temperature. When the temperature of the material drops to 0°C, start to add 1.05 mol of sulfuryl chloride dropwise. During the dropping process, keep the temperature at 0-5°C. After the addition is complete, keep warm for 0.5 hours, take a sample and control it, and the reaction is completed if the residual triethyl ester is ≤0.2%. Raise the temperature to 80°C and keep it warm for 1 hour. After the heat preservation was completed, cool to 50°C, add 100ml of water, stir for half an hour, statically separate the layers, wash the organic layer with water until neutral, and obtain ethyl chloroacetoacetate with a content of 98% and a yield of 97%.
Embodiment 2
[0026] Example 2, the synthesis of 2-chloro-2-(2,4-dichlorophenylhydrazone)-ethyl glyoxylate
[0027] Put 350ml of hydrochloric acid and 400ml of water into the reaction bottle, start stirring, then put in 100g of 2,4-dichloroaniline, cool to below 0°C (above -10°C), start to add dropwise sodium nitrite aqueous solution (44g of sodium nitrite, 100ml water), dropwise adding time 2-2.5 hours. During the whole dropping process, the temperature of the material should be kept between -5 and 0°C. The generated diazonium salt is used for later use.
[0028] Put 600ml of methanol and 200ml of water into another reaction bottle, put 400g of sodium acetate under stirring, and then add 110g of chlorotriethyl ester (ie ethyl chloroacetoacetate). The temperature was lowered to 15°C, and the diazo liquid was added dropwise, and the whole dropping time was about 1 hour. After the dropwise addition, keep the temperature for 3 hours. After the heat preservation is over, discharge the materi...
Embodiment 3
[0029] Embodiment 3, cyclization
[0030] Put 500g of ethyl methacrylate and 450g (95%) 2-chloro-2-(2,4-dichlorophenylhydrazone)-ethyl glyoxylate into the reaction bottle, then add 10g of triethylamine and 2, 2g of 5-di-tert-butylhydroquinone, raise the temperature to 60°C and start to add potassium bicarbonate solution (150g of potassium bicarbonate dissolved in 550mL of water) dropwise, and drop it in about 3 hours. After the dropwise addition, keep warm for 2.5 hours, stand still for 30 minutes, and separate the lower layer of waste water. Start to heat up, high-vacuum precipitation, the precipitation is completed, add 350mL of methanol, cool down, wait until the temperature reaches -5 ° C, centrifuge to obtain 505g of product, the content is 95.2%, and the yield is 88%. Melting point: 50∽52°C. Embodiment 4, the synthesis of ethyl chloroacetoacetate
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com