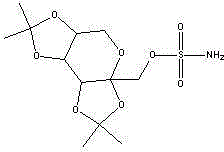
Topiramate sustained-release preparation and preparation method thereof
A technology of sustained-release preparations and topiramate, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve problems such as complex preparation processes, reduce side effects, improve adaptability, Seizure Control Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: preparation topiramate sustained-release dry suspension
[0029] Topiramate 200g
[0030] Polysulfonyl styrene sodium ion exchange resin 400g
[0031] Polyethylene glycol 168g
[0032] Ethyl cellulose 76.8g
[0033] Sucrose 2053.2g
[0034] Xanthan gum 24g
[0035] Citric acid 18g
[0036] Micropowder silica gel 30g
[0037] Flavor 30g
[0038] Make 1000 bags
[0039] Preparation Process:
[0040] 1. To prepare the drug-resin complex, add topiramate to a certain amount of water, heat to dissolve, then add polysulfonylstyrene sodium ion exchange resin, continue to stir for 1-12h, let stand, filter, and dry;
[0041] 2. Drug resin complex PEG impregnation treatment, drying;
[0042] 3. Prepare a coating solution, dissolve ethyl cellulose in a certain amount of 95% ethanol, and set aside;
[0043] 4. Coating with a fluidized bed to obtain sustained-release granules;
[0044] 5. Sucrose and citric acid were crushed to 80 mesh respectively, and micr...
Embodiment 2
[0049] Embodiment 2: Preparation of topiramate sustained-release capsules
[0050] Topiramate 200g
[0051] Polysulfonyl styrene sodium ion exchange resin 400g
[0052] Polyethylene glycol 168g
[0053] Ethyl cellulose 76.8g
[0054] Microcrystalline cellulose 80g
[0055] Micropowder silica gel 9.2g
[0056] Make 1000 capsules
[0057] Preparation Process:
[0058] 1. To prepare the drug-resin complex, add topiramate to a certain amount of water, heat to dissolve, then add polysulfonylstyrene sodium ion exchange resin, continue to stir for 1-12h, let stand, filter, and dry;
[0059] 2. Drug resin complex PEG impregnation treatment, drying;
[0060] 3. Prepare a coating solution, dissolve ethyl cellulose in a certain amount of 95% ethanol, and set aside;
[0061] 4. Coating with a fluidized bed to obtain sustained-release granules;
[0062] 5. Mix the slow-release granules with microcrystalline cellulose and micropowder silica gel evenly;
[0063] 6. Fill capsules an...
Embodiment 3
[0064] Embodiment 3: preparation topiramate sustained-release tablet
[0065] Topiramate 200g
[0066] Polysulfonyl styrene sodium ion exchange resin 400g
[0067] Polyethylene glycol 168g
[0068] Ethyl cellulose 76.8g
[0069] Microcrystalline cellulose 80g
[0070] Croscarmellose Sodium 10g
[0072] Make 1000 capsules
[0073] Preparation Process:
[0074] 1. To prepare the drug-resin complex, add topiramate to a certain amount of water, heat to dissolve, then add polysulfonylstyrene sodium ion exchange resin, continue to stir for 1-12h, let stand, filter, and dry;
[0075] 2. Drug resin complex PEG impregnation treatment, drying;
[0076]3. Prepare a coating solution, dissolve ethyl cellulose in a certain amount of 95% ethanol, and set aside;
[0077] 4. Coating with a fluidized bed to obtain sustained-release granules;
[0078] 5. Mix the sustained-release granules with microcrystalline cellulose, croscarmellose sodium and magnesiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com