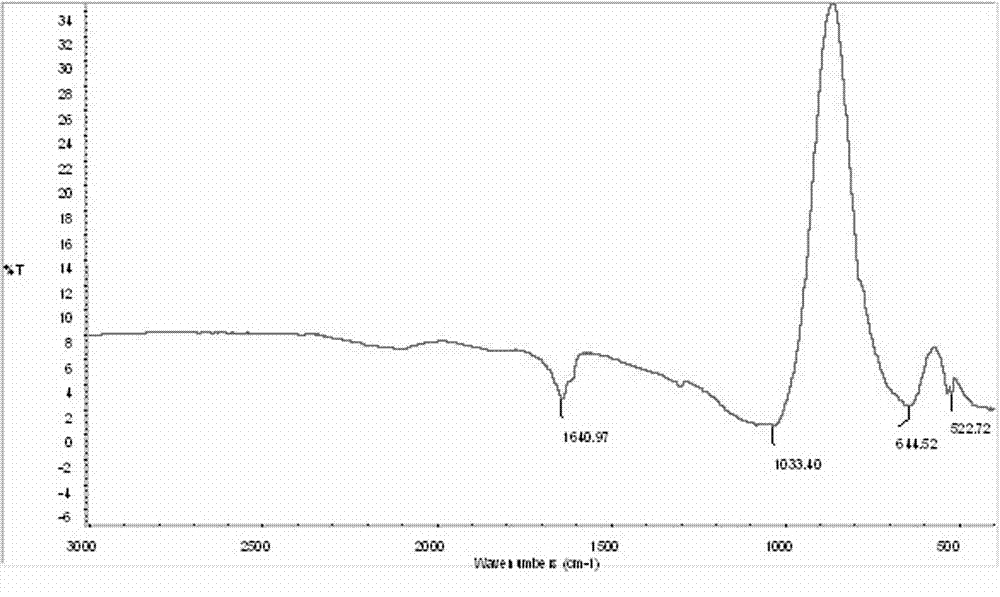
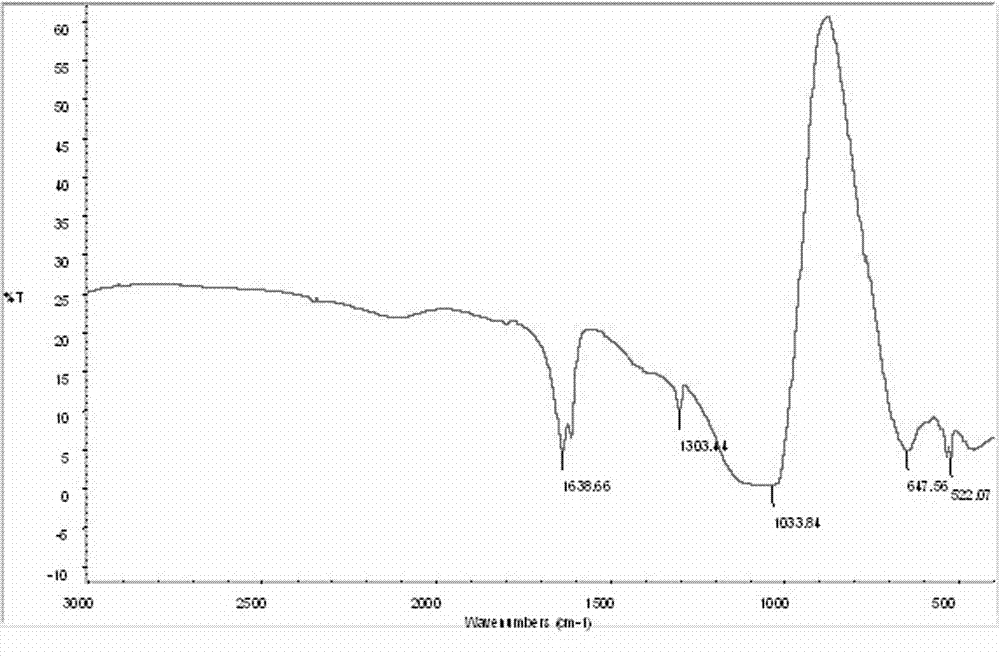
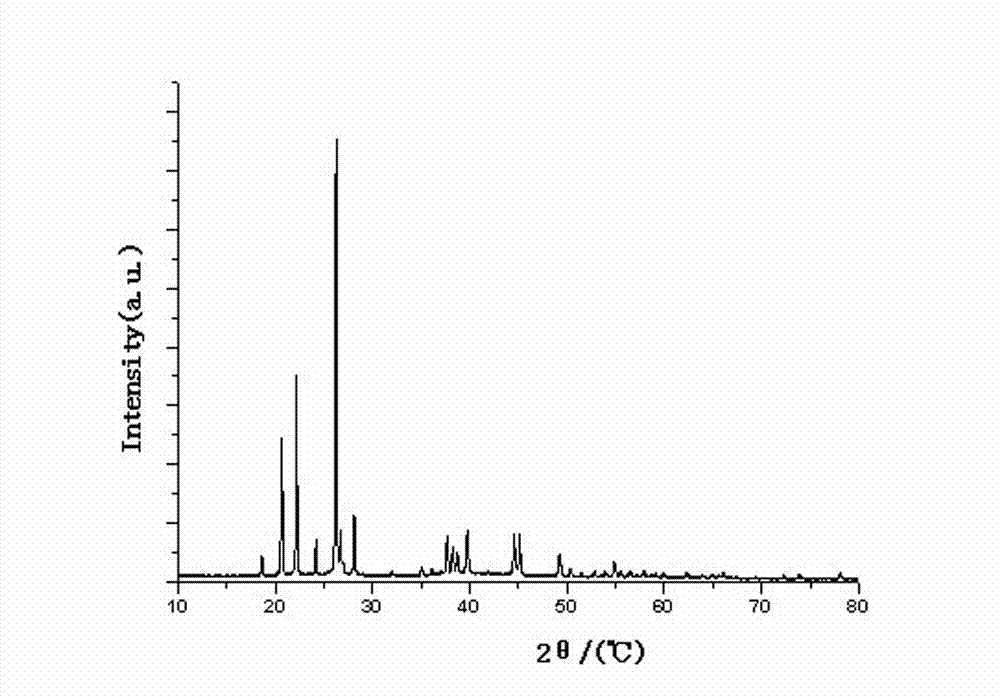
Preparation method of high-purity lithium tetrafluoroborate
A technology of lithium tetrafluoroborate and high-purity lithium fluoride, which is applied in the directions of tetrafluoroboric acid, borate, boron oxide, etc., can solve the difficulty of increasing the production control process, the low yield of lithium tetrafluoroborate, and the acidic substances in the product. Exceeding the standard and other problems, to achieve the effect of convenient transportation and storage, abundant source of raw materials, and reducing the viscosity of the system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The molar ratio of high-purity lithium fluoride to boron trifluoride complex is 1.1, the reaction temperature is 8°C, and the reaction time is 23h;
[0049] After the reaction is completed, unreacted lithium fluoride is removed by filtration, and the filtrate is heated and concentrated under vacuum or under the protection of a dry inert gas until the LiBF4 content in the concentrate is 70%;
[0050] Add a low-polarity solvent to the concentrate, extract the remaining chain carbonate organic solvent, and promote the precipitation of lithium tetrafluoroborate in the system;
[0051] After extraction and crystallization, filter, and then wash with an organic solvent to remove the residual linear carbonate solvent. The organic solvent for washing can be cyclohexane, cyclopentane, hexane, pentane, carbon tetrachloride, diethyl ether, propyl ether, butyl ether One or more mixtures of , toluene, xylene or styrene, the wet product obtained after washing is dried under the prote...
Embodiment 2
[0053] Same as Example 1, except that the molar ratio of high-purity lithium fluoride to boron trifluoride complex is 1.2, the reaction temperature is 35° C., and the reaction time is 15 h;
[0054] After the reaction is completed, unreacted lithium fluoride is removed by filtration, and the filtrate is heated and concentrated under vacuum or under the protection of a dry inert gas until the LiBF4 content in the concentrate is 65%.
Embodiment 3
[0056] Same as Example 1, except that the molar ratio of high-purity lithium fluoride to boron trifluoride complex is 1.4, the reaction temperature is 12°C, and the reaction time is 5h;
[0057] After the reaction is completed, unreacted lithium fluoride is removed by filtration, and the filtrate is heated and concentrated under vacuum or under the protection of a dry inert gas until the LiBF4 content in the concentrate is 45%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| relative humidity | aaaaa | aaaaa |
| Bronsted acidity | aaaaa | aaaaa |
| relative humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com