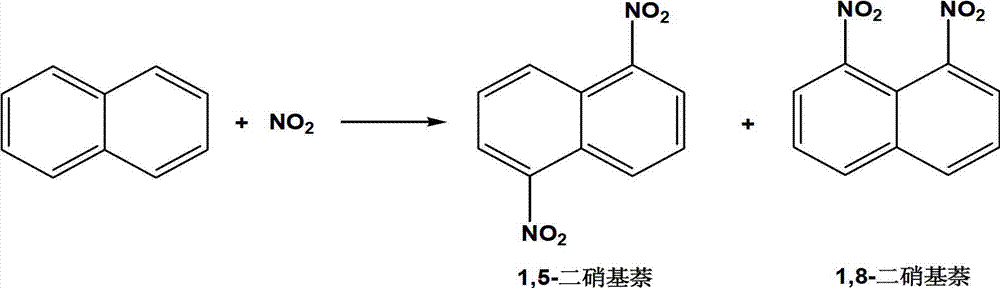
Preparation method of 1,5-dinitronaphthalene and 1,8-dinitronaphthalene
A dinitronaphthalene and nitrogen dioxide technology, applied in the field of synthesis of 1,5-dinitronaphthalene and 1,8-dinitronaphthalene, can solve poor safety performance, low atom economy and environmental pollution Serious problems, to achieve the effect of increasing yield and selectivity, improving atom utilization, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
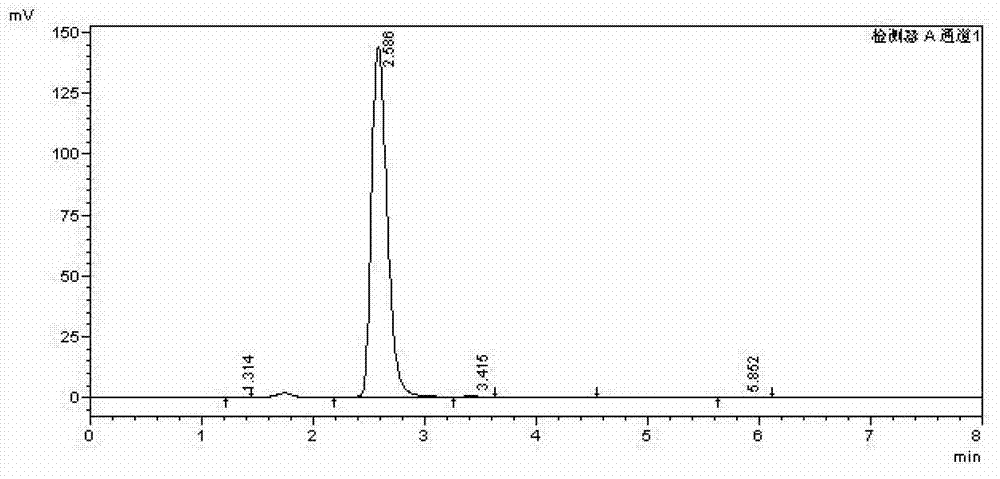
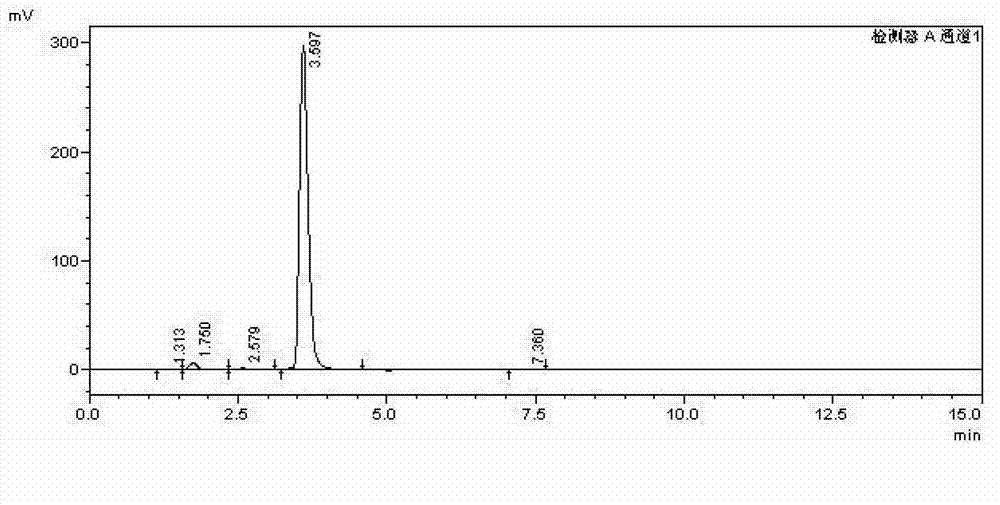
[0021] In a 50 mL three-necked flask, add 0.128 g of naphthalene, 5.0 mL of acetonitrile, 0.100 g of HZSM-11, 2.0 mL of nitrogen dioxide, stir magnetically in an oxygen atmosphere, and react at room temperature for 12 hours. After the reaction time has elapsed, the reaction is terminated with deionized water. The mixture was filtered through a glass funnel to remove the catalyst. The filtrate was rotary evaporated in vacuo to obtain a crude product, which was washed three times with 5% aqueous sodium bicarbonate solution, and then washed with distilled water until the crude product was neutral. Nitrobenzene was used as internal standard for HPLC analysis, and the content of each isomer component of nitration products was calculated by internal standard method. The mass of 1,5-dinitronaphthalene and 1,8-dinitronaphthalene is 0.160g, and the yield is 73.0%, of which the mass of 1,5-dinitronaphthalene is 0.109g, and the mass fraction is 68.1%; 1 , The mass of 8-dinitronaphthale...
Embodiment 2
[0024] In a 50 mL three-necked flask, add 0.128 g of naphthalene, 5.0 mL of acetonitrile, 0.100 g of Hβ-500, and 2.0 mL of nitrogen dioxide, stir magnetically in an oxygen atmosphere, and react at room temperature for 12 hours. After the reaction time has elapsed, the reaction is terminated with deionized water. The mixture was filtered through a glass funnel to remove the catalyst. The filtrate was rotary evaporated in vacuo to obtain a crude product, which was washed three times with 5% aqueous sodium bicarbonate solution, and then washed with distilled water until the crude product was neutral. After drying, carry out HPLC analysis with nitrobenzene as the internal standard, and calculate the content of each isomer component of the nitration product with the internal standard method. The mass of 1,5-dinitronaphthalene and 1,8-dinitronaphthalene is 0.177g, and the yield is 81.4%, of which the mass of 1,5-dinitronaphthalene is 0.131g, and the mass fraction is 74.0%; 1 , The...
Embodiment 3
[0027] In a 50 mL three-necked flask, add 0.128 g of naphthalene, 5.0 mL of acetonitrile, 0.200 g of HY, and 2.0 mL of nitrogen dioxide, stir magnetically in an oxygen atmosphere, and react at room temperature for 12 hours. After the reaction time has elapsed, the reaction is terminated with deionized water. The mixture was filtered through a glass funnel to remove the catalyst. The filtrate was rotary evaporated in vacuo to obtain a crude product, which was washed three times with 5% aqueous sodium bicarbonate solution, and then washed with distilled water until the crude product was neutral. After drying, carry out HPLC analysis with nitrobenzene as internal standard, and calculate the content of each isomer component after nitration of naphthalene with internal standard method. The mass of 1,5-dinitronaphthalene and 1,8-dinitronaphthalene is 0.139g, and the yield is 63.8%. Among them, the mass of 1,5-dinitronaphthalene is 0.096g, and the mass fraction is 69.1%; the mass o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com