Dasatinib dispersoid, preparation method thereof and application thereof in tablets
A dasatinib and dispersion technology, applied in the field of pharmaceutical preparations, can solve the problems of low bioavailability, incomplete drug release, and easy to appear sticky, etc., to reduce production costs, improve bioavailability, and be easy to take Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
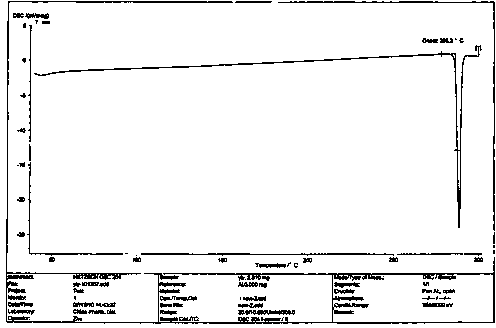
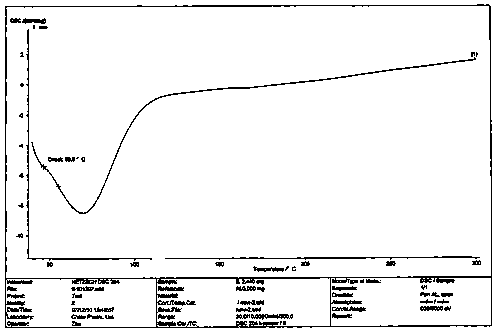
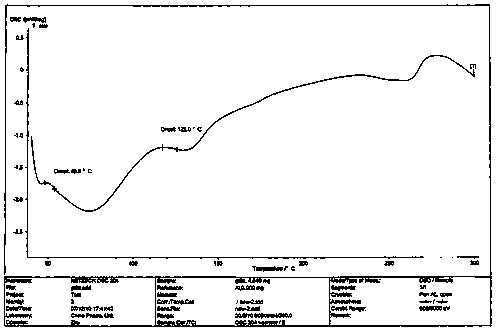
Image
Examples
Embodiment 1
[0044]The preparation of dasatinib solid dispersion, the specific steps are: take dasatinib and polyvinylpyrrolidone PVP with a mass ratio of 1: (0.5-10), and fully dissolve them in N, N -Dimethylformamide (DMF) solution, the solution is weighed according to the amount of 1 part of Dasatinib by mass and 4 to 30 parts by volume of the solution. After the dissolution is complete, the obtained solution is slowly dropped into the stirring In the precipitant mixed with ethyl acetate or ethyl acetate and petroleum ether, the volume ratio of the solution to the precipitant is 1: (5-40). With the continuous dripping of the solution, the precipitate is continuously precipitated from the precipitant until the precipitation is complete. Finally, filter out the precipitate, vacuum filter to remove the organic solvent contained in the precipitate, then place it in an oven at 50-60°C for drying, and sieve the dry powder to obtain the Dasatinib solid dispersion. Partition, the detailed selec...
Embodiment 2
[0058] The preparation of dasatinib solid dispersion tablets specifically includes: (1) weighing the prescribed amount of dasatinib solid dispersion and tablet excipients; Select the appropriate punch for the required specifications, and test the tablet to adjust the hardness; (4) Compress the tablet; (5) Coat with 3% Opadry ethanol solution, and the weight of the coating increases by 3%. In different tablet specifications, the detailed selection ratio of each component and solution is as follows, but the preparation ratio is not limited to the following:
[0059] 1.1 Tablet Specification Tablet weight 160mg, dasatinib solid dispersion 40mg, of which dasatinib content is 20mg, microcrystalline cellulose 105.6mg, croscarmellose sodium 12.8mg, magnesium stearate 1.6mg . 3% Opadry ethanol solution coating weight gain 3%. The tablet hardness of this specification is 13.35kg.
[0060] 1.2 Tablet specifications Tablet weight 400mg, dasatinib solid dispersion 100mg, of which dasat...
Embodiment 3
[0064] In vitro dissolution test, in vitro dissolution test according to the dissolution test regulations of the Chinese Pharmacopoeia 2010 edition, using the paddle method and the big cup method, the test time is 2 hours, the speed is 50rpm in the first hour, 200rpm in the next hour, and the temperature is 37℃±0.5 ℃, the dissolution medium is 0.1M hydrochloric acid. The specifications obtained by detecting Example 2 respectively are tablet weight 160mg-containing drug 20mg, tablet weight 400mg-containing drug 50mg, tablet weight 400mg-containing drug 70mg, tablet weight 500mg-containing drug 100mg tablet in vitro dissolution rate, the dissolution rate 106.8%, 98.8%, 99.8%, 97.8% respectively, the dissolution curve is shown in Figure 3-Figure 6 , each tablet met the dissolution rate standard.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com