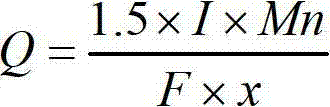Method for preparing high-purity peroxysulphate based on electrolytic oxidation through ion-exchange membrane electrolyzer for chlor-alkali production
A chlor-alkali ion membrane, persulfate technology, applied in the electrolysis process, electrolysis components, organic diaphragms and other directions, can solve the problems of low current efficiency, low production efficiency, caustic soda consumption, etc., to achieve high current efficiency, low power consumption, resistance to Strong electrochemical corrosion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
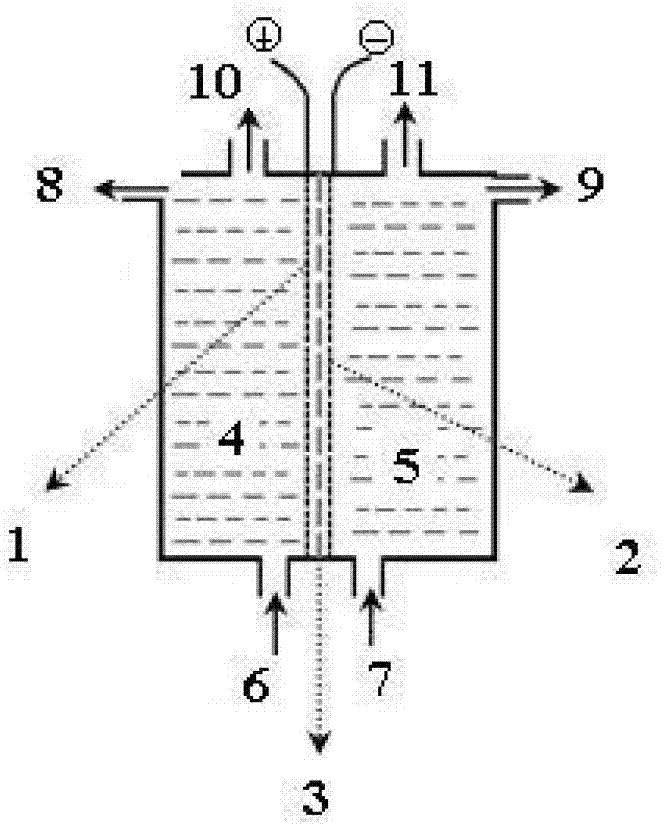
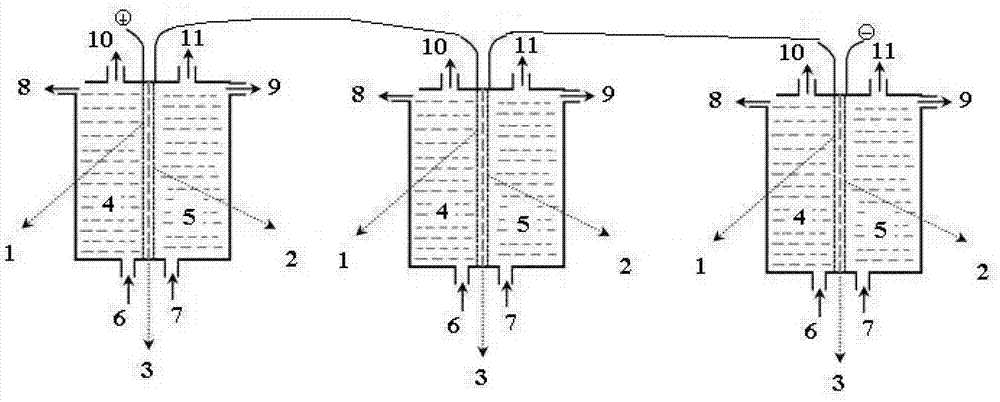
[0066] A method for preparing high-purity persulfate by electrolysis, by using an electrolytic electrolysis method in which a perfluorinated ion exchange membrane 3 is arranged between electrodes and is mainly composed of an anode chamber 4, an anode 1, a perfluorinated ion exchange membrane 3, a cathode 2, and a cathode chamber 5. slot, proceed as follows:
[0067] (1) in such as figure 1 Electrochemical ion exchange is carried out in the ionic membrane electrolyzer shown, the cathode electrode is a nickel-based mesh electrode with active coating, the anode electrode is a titanium-based mesh electrode, and the fluoride ion exchange membrane 3 is DF988 type chlor-alkali perfluorinated Carboxylic acid-perfluorosulfonic acid composite membrane ion membrane, effective area 50cm 2 ;
[0068] (2) the anolyte raw material is an aqueous solution of 30% mass concentration ammonium sulfate, and the catholyte raw material is 28% mass concentration ammonium hydroxide solution; the anod...
Embodiment 2
[0074] (1) in such as figure 1 Electrochemical ion exchange is carried out in the ionic membrane electrolyzer shown, the cathode electrode is a stainless steel-based mesh electrode with an active coating, the anode electrode is a titanium-based mesh electrode, and the perfluorinated ion exchange membrane 3 is a DF2801 type chlor-alkali all-purpose electrode. Fluorocarboxylic acid-perfluorosulfonic acid composite membrane ion membrane, effective area 50dm 2 ;
[0075] (2) The anolyte is an aqueous solution of sodium sulfate, and the catholyte is a sodium hydroxide solution; first preheat the raw materials of the anolyte and catholyte to 35°C, and then pump the anolyte and catholyte into the anode chamber of the electrolytic cell and in the cathode compartment;
[0076] (3) At 45°C, apply direct current to the electrolytic cell for constant current electrolysis, with a current density of 3.5kA / m 2 , the sulfate mass concentration of the anolyte entering the tank is controlled...
Embodiment 3
[0080] Same as Example 1, the difference is that the current density in step (3) is 3.5kA / m 2 .
[0081] After data processing, the current efficiency of this process is 98.47%, the power consumption is 2210kWh / t, and the purity of the product ammonium persulfate is >99.72%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| power consumption | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com