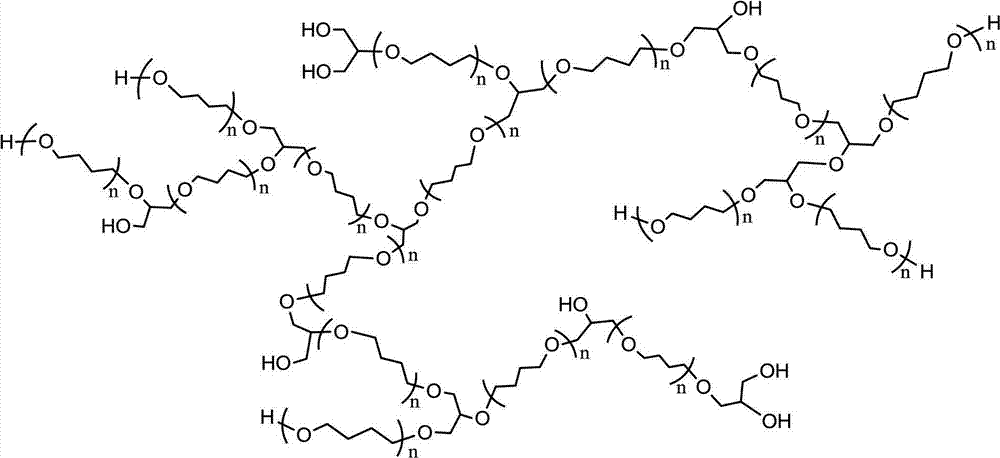
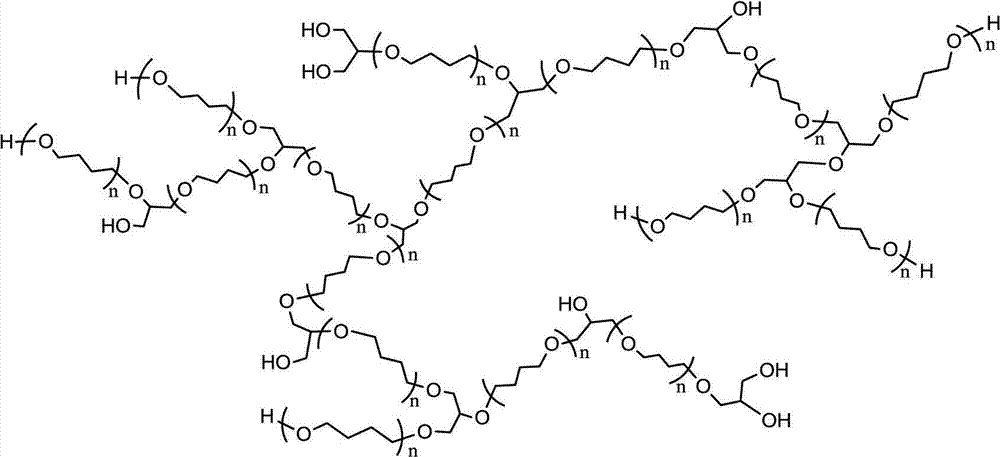
Tetrahydrofuran-glycidol random hyperbranched copolyether and preparation method thereof
A technology of tetrahydrofuran and glycidol, which is applied in the field of tetrahydrofuran-glycidol random hyperbranched copolyether and its preparation, can solve the problems of high degree of branching of products and difficulty in controlling the degree of branching of polymers , to achieve the effect of wide sources, simple and easy methods, and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
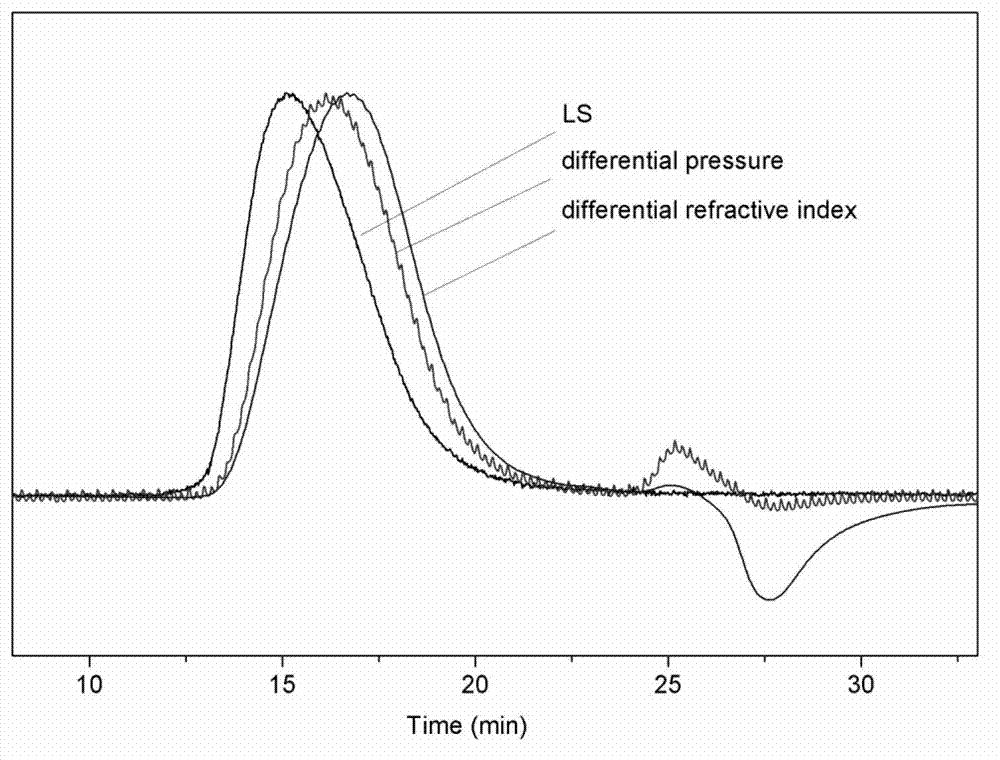
[0021] Add 10mL of tetrahydrofuran and 3mL of glycidol into a 100ml round bottom flask. After stirring for 10 minutes in an ice-water mixed bath, 0.1 mL of boron trifluoride tetrahydrofuran complex was added to react for 2 hours. Finally, 10 mL of water was added to terminate the reaction, and after stirring for 15 minutes, the mixture was evaporated to dryness to obtain a crude product. The crude product was purified by dialysis with a molecular weight cut-off of 300, and a colorless viscous liquid was obtained after drying. The product was characterized by Gel Permeation Chromatography-Laser Light Scattering-Viscosity Detector (such as figure 1 shown): Mn=8195, Mw=11640, Mw / Mn=1.42; the parameters of the Mark-Houwink-Sakurada equation are:
[0022] K:(5.639±0.078)×10 -1 mL / g
[0023] a:0.334±0.001
[0024] Where a is less than 0.5, indicating that the prepared polymer product has typical hyperbranched structure characteristics.
[0025] Proton nuclear magnetic spectrum...
Embodiment 2
[0027] 10 mL of tetrahydrofuran and 2 mL of glycidol were added to a 100 ml round bottom flask. After stirring for 15 minutes in an ice-water mixed bath, 0.18 g of phosphotungstic acid was added and reacted for 3 hours. Finally, 10 mL of 2% sodium hydroxide solution was added to terminate the reaction, stirred for 15 minutes and then evaporated to dryness to obtain a crude product. The crude product was characterized by gel permeation chromatography-laser light scattering (SEC-MALLS): Mn=6689, Mw=12200, Mw / Mn=1.824. From the elution curve of the test, it can be found that the product contains some oligomers of small molecules. The crude product was purified by dialysis for 36 hours in a dialysis bag with a molecular weight cut-off of 300. After drying, a colorless viscous product was obtained. Characterized by SEC-MALLS: Mn=11610, Mw=14540, Mw / Mn=1.196.
Embodiment 3
[0029] 10 mL of tetrahydrofuran and 3 mL of glycidyl alcohol were added to a 100 ml round bottom flask. After stirring for 15 minutes at minus 10°C, add 1 mL of acetic anhydride and 0.15 mL of perchloric acid. After reacting for 3 hours, add 10 mL of water to terminate the reaction. After stirring for 15 minutes, evaporate to dryness to obtain a colorless thick product. The crude product was dissolved in water and purified by dialysis with a dialysis bag with a molecular weight cut-off of 300. After drying, a colorless viscous liquid was obtained, characterized by SEC-MALLS: Mn=2266, Mw=2887, Mw / Mn=1.274).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com