Preparation method of fluoropyridine compounds
A fluorine-containing pyridine and compound technology is applied in the field of preparation of pharmaceutical intermediates, which can solve the problems of single application object, inability to use large-scale mass production, etc., and achieve the effects of reducing damage and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
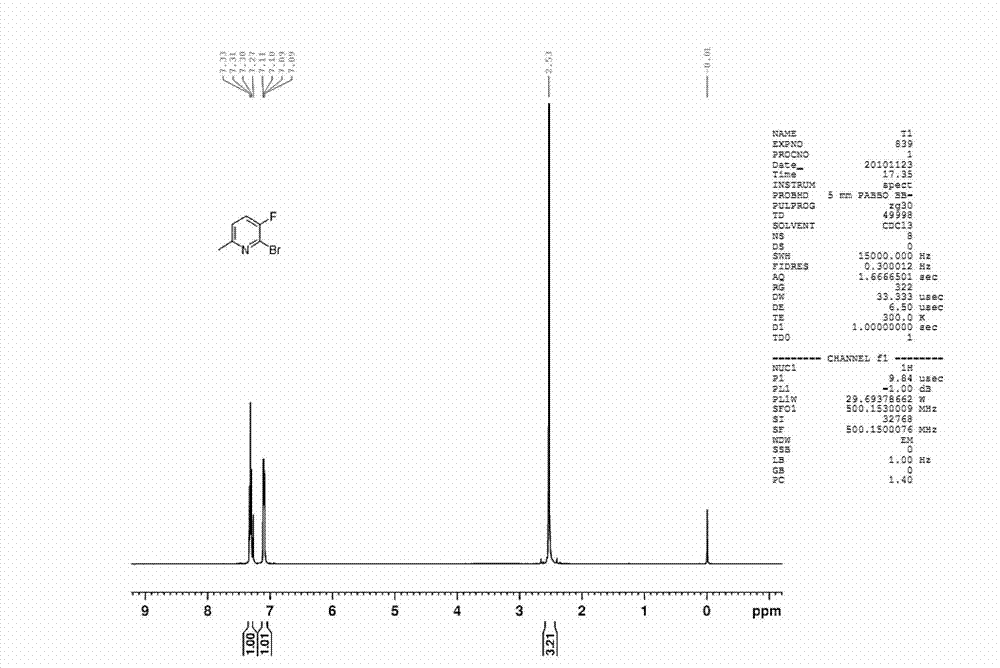
[0034] Preparation of 2-bromo-3-fluoro-6-methylpyridine
[0035] synthetic route:
[0036]
[0037] making process:
[0038] The first step: under ice bath conditions, 3-amino-6-picoline, compound A1 (22.6g, 0.209mol, 1.0eq) was added to 150mL of acetonitrile, and sodium bromide was added thereto under stirring and sodium bromate aqueous solution 100mL (containing sodium bromide 0.139mol, 14.3g, containing sodium bromate 0.070mol, 10.6g), after adding, concentrated sulfuric acid (0.314mol, 30.7g, 1.5eq) was added dropwise to it aqueous solution (80mL) and kept in cold conditions. After reacting at room temperature for 3 hours, a saturated sodium bicarbonate solution was added to the reaction solution until the reaction solution became neutral. The reaction solution was extracted with ethyl acetate (150 mL*3). The organic phase was dried with anhydrous sodium sulfate, filtered, spin-dried, and recrystallized with ethyl acetate / petroleum ether system to obtain 3-amino-2-b...
Embodiment 2
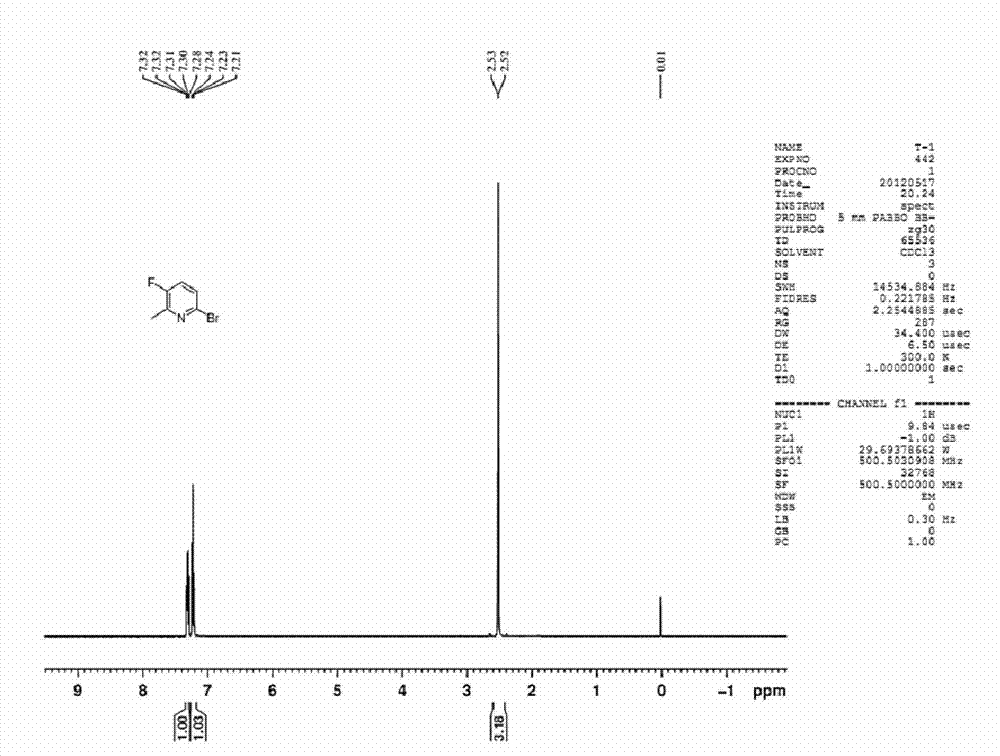
[0042] Preparation of 2-bromo-5-fluoro-6-methylpyridine
[0043] synthetic route:
[0044]
[0045] making process:
[0046] The first step: 2-hydroxyl-5-nitro-6-picoline, compound B1 (26.8g, 0.174mol, 1.0eq) was added to 120mL acetonitrile, and slowly added phosphorus oxybromide ( 99.8g, 0.348mol, 2.0eq), after the addition, the reaction temperature was raised to 110°C-130°C, and the reaction was continued for 3 hours. The reaction solution was cooled and slowly added to an ice-water mixture to quench, adjusted to neutrality with saturated sodium bicarbonate solution, extracted with dichloromethane (150mL*3), dried with anhydrous sodium sulfate, filtered, and spin-dried to obtain 2-bromo-6-methyl-5-nitropyridine, 35.0 g of the crude product of compound B2, the yield is 92.8%
[0047] The second step: the crude product B2 (35.0 g, 0.161 mol) was added to 150 ml of methanol, 15 g of Raney nickel was added thereto, and reacted at room temperature under hydrogen pressure (40 ...
Embodiment 3
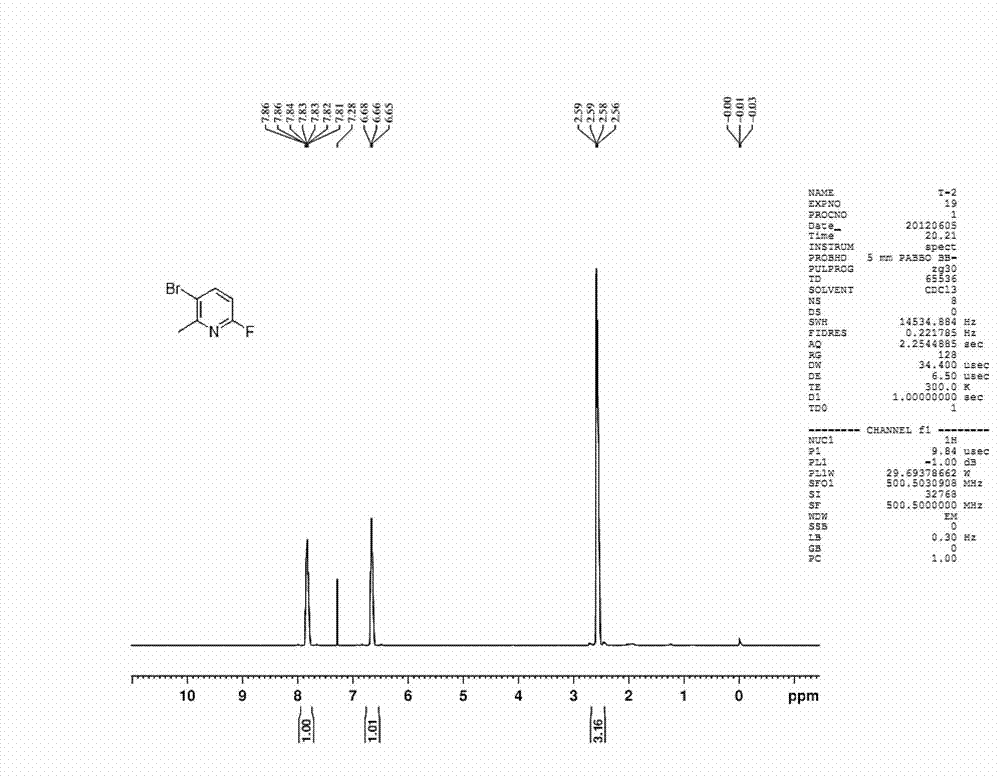
[0051] Preparation of 5-bromo-2-fluoro-6-methylpyridine
[0052]
[0053] making process:
[0054] The first step: under ice-bath conditions, 2-amino-6-picoline, compound C1 (27.3g, 0.253mol, 1.0eq) was added to 180mL of acetonitrile, and sodium bromide was added thereto under stirring and sodium bromate aqueous solution 100mL (containing sodium bromide 0.169mol, 17.4g, containing sodium bromate 0.084mol, 12.7g), after adding, concentrated sulfuric acid (0.380mol, 37.2g, 1.5eq) was added dropwise to it aqueous solution (80mL) and kept in cold conditions. After reacting at room temperature for 3 hours, a saturated sodium bicarbonate solution was added to the reaction solution until the reaction solution became neutral. The reaction solution was extracted with ethyl acetate (150 mL*3). The organic phase was dried with anhydrous sodium sulfate, filtered, spin-dried, and recrystallized with ethyl acetate / petroleum ether system to obtain 5-bromo-2-amino-6-picoline, namely com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com