Fluorescent labeling polyaspartic acid scale inhibitor and preparation method thereof
A polyaspartic acid, fluorescent labeling technology, applied in chemical instruments and methods, descaling and water softening, water/sludge/sewage treatment, etc. The problem of serious interference, etc., can achieve the effect of online regulation of chemical concentration, reduction of use cost, and improvement of scale inhibition performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
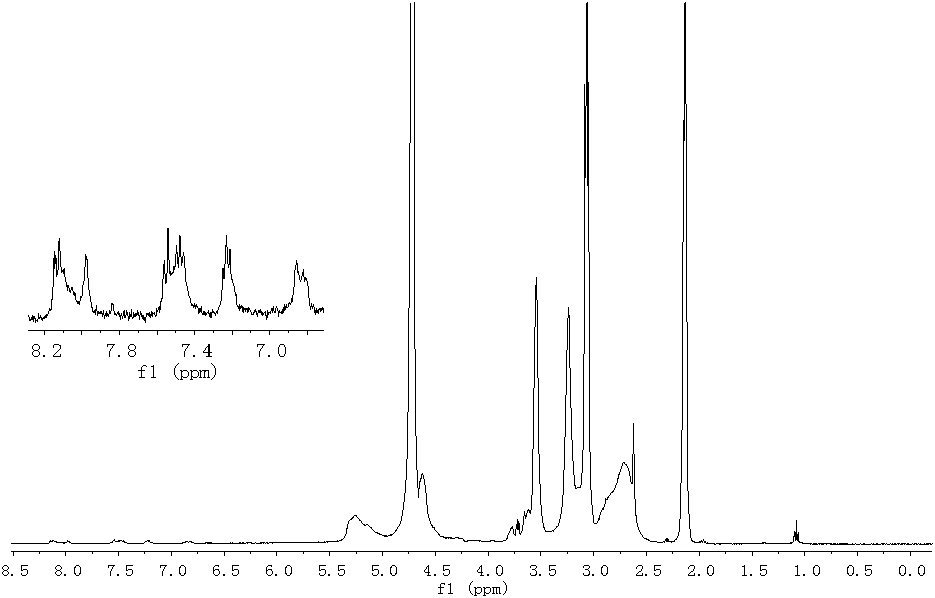
[0026] Add 2g (15.67mmol) of hydroxylated polysuccinimide (polysuccinimide synthesized by condensation of aspartic acid and monoethanolamine) into a dry three-necked round-bottomed flask. The molar ratio of the amine structural unit is 1:1) and 6g of sulfolane. After heating it to completely dissolve, add 0.21g (0.94mmol) N-(2,3-epoxypropyl) carbazole and 0.4mL Tris Boron fluoride ether, at 75 ° C for 5h. After the end, it was slowly poured into constantly stirring dehydrated ethanol to precipitate out, suction filtered, washed 3 times with dehydrated ethanol, and dried at 80°C to obtain 2.12 grams of light brown solid fluorescently labeled polysuccinimide, the yield 95.9%. 1 H-NMR (400MHz, D 2 O, TMS) as attached figure 1 Shown, δ: 8.09 (s, 2H, ArH4), 7.50 (m, 2H, ArH1), 6.84 ~ 7.21 (m, 2H, ArH2 and ArH3), 5.26 (m, 1H, NHCHCO), 4.61 ~ 4.65 (m , 3H, ArNCH 2 CH(OH)CH 2 O), 3.55~3.77(m, 4H, CH(OH)CH 2 OCH 2 CH 2 NH), 3.05~3.24(m, 2H, CH 2 CO), 2.62~2.69(m, 2H, CH(OH)CH...
Embodiment 2
[0028] Add 2g (15.67mmol) of hydroxylated polysuccinimide (polysuccinimide synthesized from maleic anhydride and ammonium carbonate and monoethanolamine) into a dry three-necked round-bottomed flask. The imine structural unit molar ratio is 1:1) and 6g sulfolane, after it is heated to dissolve completely, then add 0.21g (0.94mmol) N-(2,3-epoxypropyl) carbazole, 0.4mL trifluoro Boronium ether was reacted at 75°C for 5h under a nitrogen atmosphere. After the end, it was slowly poured into constantly stirring dehydrated ethanol to precipitate out, suction filtered, washed 3 times with dehydrated ethanol, and dried at 80°C to obtain 2.09 grams of light brown solid fluorescently labeled polysuccinimide, the yield 94.6%.
Embodiment 3
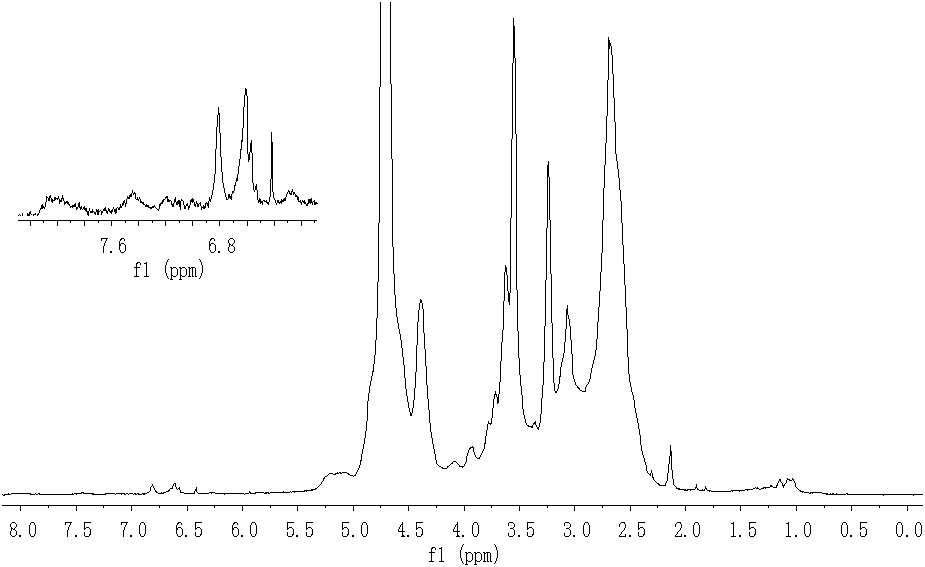
[0030]Weigh 1.7g (12.57mmol) of the fluorescently labeled polysuccinimide prepared in Example 1, place it in a 50mL round bottom flask, add 3.2mL of 2mol / L sodium hydroxide solution, and react at 50-60°C After 20 minutes, the pH value was adjusted to below 7 with concentrated hydrochloric acid after the hydrolysis. Slowly pour it into 15 mL of dehydrated ethanol that is constantly stirring, precipitate out, filter with suction, wash 3 times with dehydrated ethanol / diethyl ether (volume ratio 1:1), and vacuum-dry at 60°C to obtain 1.79 grams of light brown fluorescent marker Polyaspartic acid, yield 98.7%. 1 H-NMR (400MHz, D 2 O, TMS) as attached figure 2 As shown, δ: 8.01 (s, 2H, ArH4), 7.42 (m, 2H, ArH1), 6.28~6.81 (m, 4H, ArH2 and ArH3), 5.15 (m, 1H, NHCHCO), 4.39 (m, 3H , ArNCH 2 CH(OH)CH 2 O), 3.55~3.77(m, 4H, CH(OH)CH 2 OCH 2 CH 2 NH), 3.06~3.24(m, 2H, CH 2 CO), 2.67(m, 2H, CH(OH)CH 2 OCH 2 CH 2 NH), 2.14 (s, 1H, OH).
[0031] The fluorescently labeled polya...
PUM
| Property | Measurement | Unit |
|---|---|---|
| scale inhibition rate | aaaaa | aaaaa |
| scale inhibition rate | aaaaa | aaaaa |
| scale inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com