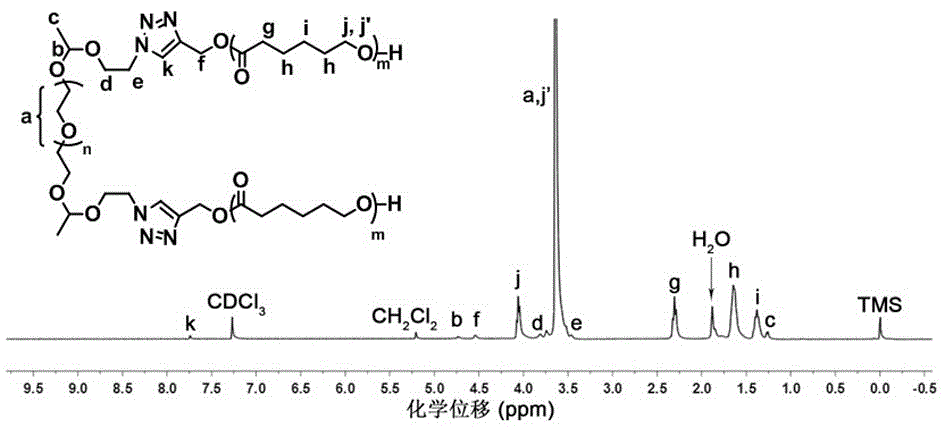
Amphiphilic triblock copolymer with acid sensitivity and preparation method and application thereof
A sensitive, three-block technology, applied in the direction of medical preparations and pharmaceutical formulations of non-active ingredients, can solve the problems of limiting the application of anticancer drugs, poor water solubility, and large toxic and side effects, and achieve high yield and excellent reaction conditions Simple, good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
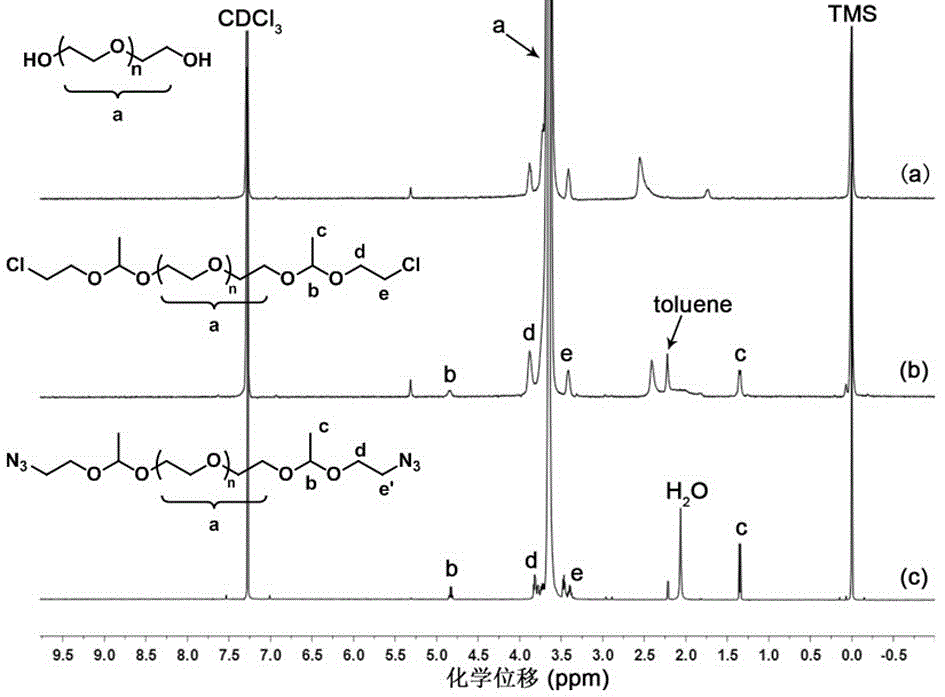
[0058] Embodiment one: Polyethylene glycol (Cl- α -PEG 135 - α -Cl) synthesis.
[0059] First, through double-terminal hydroxyl-containing polyethylene glycol (HO-PEG 135- OH) and pyridinium p-toluenesulfonate (PPTS) in toluene to remove water by azeotropy, and then use anhydrous dichloromethane as solvent to react with vinyl chloride ethyl ether (CEVE) to prepare terminal dichloroethyl biscondensate Aldehyde polyethylene glycol (Cl- α -PEG 135 - α -Cl). The specific synthesis method is as follows:
[0060] Add polyethylene glycol (6.01 g, 1.0 mmol), pyridinium p-toluenesulfonate (PPTS, 0.5 g, 0.2 mmol) into a 100 mL round bottom flask equipped with a magnetic stirring bar, a drying tube and an atmospheric distillation device and 30 mL of toluene, heated to azeotrope, and distilled out the azeotrope of toluene and water at 110 °C.
[0061] will be loaded with HO-PEG 135 -OH and PPTS round-bottomed flask was cooled to room temperature and added anhydrous dichlorometha...
Embodiment 2
[0063] Embodiment two: the polyethylene glycol (N 3 - α -PEG 135 - α -N 3 )Synthesis.
[0064]In a 50 mL round bottom flask, add Cl- α -PEG 135 - α -Cl (5.03 g, 0.8 mmol), sodium azide (0.57 g, 8 mmol) and 10 mL of dimethylformamide (DMF). The reaction was carried out at 60 °C for 40 h. The reaction system was passed through a basic aluminum oxide column (Al 2 o 3 ) to remove unreacted sodium azide, then use a vacuum oil pump to remove DMF at 50 °C, add 60 mL CH 2 Cl 2 After dilution, wash twice with PBS buffer (10 mL) at pH 10.0, separate the liquids and let stand, collect the organic phase, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate in ice n-hexane and ice ether (volume ratio 1:1) precipitation, filtration, repeated three times, pumped to dryness, and then placed in a vacuum oven to dry for 24 hours, to obtain polyethylene glycol (N 3 - α -PEG 135 - α -N 3 , 2.48 g), yield 49%.
Embodiment 3
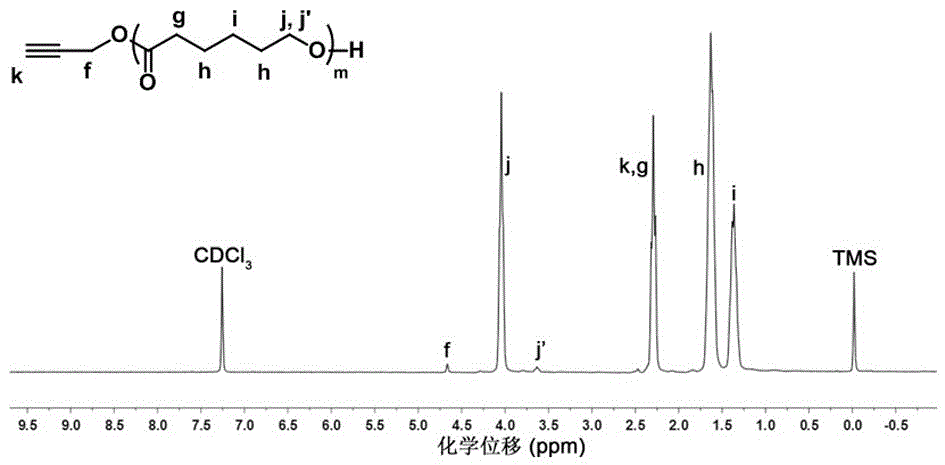
[0065] Example 3: Polycaprolactone (PCL) with an alkynyl group at the single end 30 -C≡C) synthesis method.
[0066] Dry the branched round-bottomed flask with a stirrer in an oven at 120 °C for at least 24 h, then plug it with a glass stopper, connect it to an oil pump through a latex tube, and pump it to room temperature, then introduce high-purity argon gas, and vacuumize, repeat this process three times.
[0067] Using a dry syringe, inject propargyl alcohol (0.12 g, 2.14 mmol), caprolactone (ε-CL) (10.1 g, 86.1 mmol) and stannous octoate [Sn(Oct) 2 ] (430 mg) (calculate the dosage of ε-CL according to the theoretical degree of polymerization; [ε-CL]: [Sn(Oct) 2 ] = 1: 0.04, mass ratio), 25 mL of anhydrous toluene as solvent. After the reaction flask was filled with argon, the reaction was stirred in an oil bath at 90 °C for 4 h.
[0068] After the reaction was finished, the toluene solvent was removed by rotary evaporation, precipitated and filtered in ice anhydrous e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com