Chiral tetra-amino aniline ligand, aluminum compound thereof, preparation method and application
The technology of tetradentate aminoaniline and aminoaniline is applied in the field of chiral tetradentate ligand and two kinds of catalysts for ring-opening polymerization of lactide, and achieves various structural changes, high catalytic activity and stereoselectivity, and a wide range of applications. narrow effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0047] The present invention is further described below through specific examples, but the present invention is not limited thereto, and the specific protection scope is shown in the claims.
[0048] Preparation of Chiral Aminoanilino Ligands
Embodiment 1
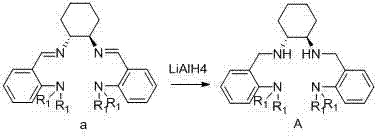
[0050] Take N,N-dimethylbenzaldehyde (10.6 g, 71 mmol) dissolved in 25 ml hexane, slowly add (1R,2R)-cyclohexanediamine (4.0 g, 35.5 mmol) dropwise, after the dropwise addition The reaction was stirred at reflux for 10 hours. Cooling and crystallization, filtering to obtain light yellow powder Schiff base a 10.3 g, 77% yield.
Embodiment 2
[0052] in N 2 Under the atmosphere, take the Schiff base (7.2 g, 19.1 mmol) of Example 1 and dissolve it in 50 ml of dry diethyl ether, slowly add lithium aluminum hydride (0.74 g, 19.1 mmol) in small amounts at 0 °C, and after the addition is complete The reaction was slowly warmed to room temperature and stirred for 8 hours. After the reaction, slowly add 1.3 mL of ice water to the reaction system to stop the reaction, then add 1.3 mL of NaOH (3M) aqueous solution, 3.9 mL of water, filter, wash the filter cake with 30 ml of ethyl acetate, collect the filtrate, and remove the solvent in vacuo to obtain pale yellow liquid A 6.6 g, 91% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com