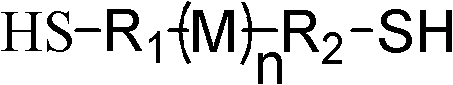Preparation method of double-mercapto-terminal polymer
A technology of polymers and mercapto groups, which is applied in the field of preparation of double-ended mercapto polymers, can solve the problems of affecting the average functionality of polymers, affecting application performance, and increasing viscosity, and achieves good industrial application prospects and easy molecular weight.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of tert-butyldimethylsilylthiopropyl chloride
[0046] In a 250ml three-necked flask, 55.0g (0.5mol) of 3-chloro-1-propanethiol, 75.3g (0.5mol) of tert-butyldimethylsilyl chloride, and 100ml of N,N-dimethylformamide were successively added. Then 37.4g (0.55mol) of imidazole was dissolved in 40ml of N,N-dimethylformamide, dropped into the three-necked flask through a constant pressure funnel, and the dropwise addition was completed within 1 hour at 0°C, and the reaction system continued to react for 24 hours. Then wash 3 times with 10% sodium bicarbonate solution, extract with petroleum ether, separate the petroleum ether layer, evaporate the solvent petroleum ether on a rotary evaporator, and separate through a chromatographic column to obtain the intermediate tert-butyldimethylsilylsulfide Propyl chloride, yield 95%. This reagent is stored in a brown bottle filled with calcium hydride.
Embodiment 2
[0047] Embodiment 2: Preparation of tert-butyldiphenylsilylthiopropyl bromide
[0048] In a 250ml three-necked flask, 77g (0.5mol) of 3-bromo-1-propanethiol, 137.5g (0.5mol) of tert-butyldiphenylchlorosilane, and 100ml of N,N-dimethylformamide were sequentially added. Then 37.4g (0.55mol) of imidazole was dissolved in 40ml of N,N-dimethylformamide, dropped into the three-necked flask through a constant pressure funnel, and the dropwise addition was completed within 1 hour at 0°C, and the reaction system continued to react for 24 hours. Wash 3 times with 10% sodium bicarbonate solution, extract with petroleum ether, separate the petroleum ether layer, evaporate the solvent petroleum ether on a rotary device, and separate through a chromatographic column to obtain the intermediate tert-butyldiphenylsilylsulfanyl Propyl bromide, yield 90%. This reagent is stored in a brown bottle filled with calcium hydride.
Embodiment 3
[0049] Embodiment 3: the preparation of triethylsilylthiopropyl bromide
[0050]In a 250ml three-necked flask, 77g (0.5mol) of 3-bromo-1-propanethiol, 75g (0.5mol) of triethylchlorosilane, and 100ml of N,N-dimethylformamide were sequentially added. Then 37.4g (0.55mol) of imidazole was dissolved in 40ml of N,N-dimethylformamide, dropped into the three-necked flask through a constant pressure funnel, and the dropwise addition was completed within 1 hour at 0°C, and the reaction system continued to react for 24 hours. Wash 3 times with 10% sodium bicarbonate solution, extract with petroleum ether, separate the petroleum ether layer, evaporate the solvent petroleum ether on a rotary device, and separate through a chromatographic column to obtain the intermediate triethylsilylthiopropyl bromide , yield 91%. This reagent is stored in a brown bottle filled with calcium hydride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com