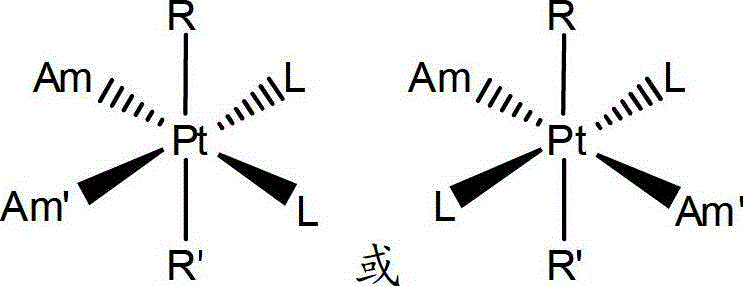
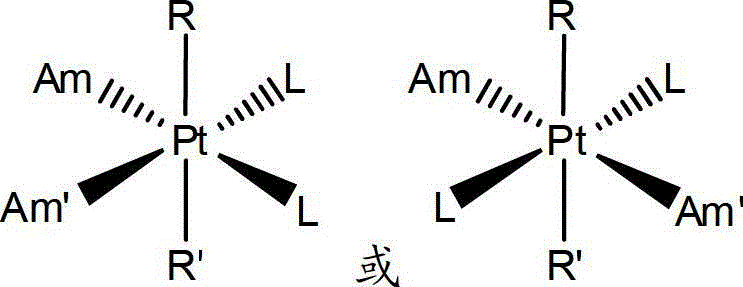
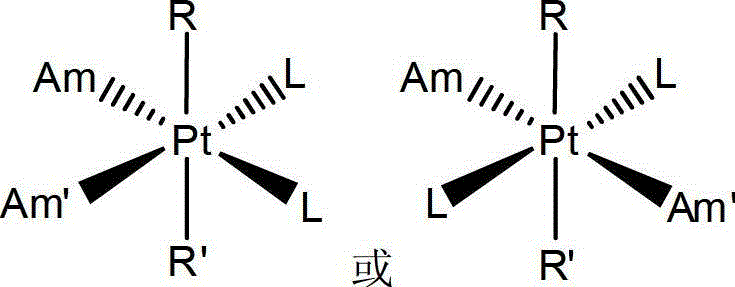
Anti-cancer medicinal aspirin platinum complex and preparation method thereof
An aspirin and complex technology, which is applied in the field of anticancer medicinal aspirin tetravalent platinum complex and its preparation, can solve the problems of unsatisfactory antitumor effect, increased fat solubility, large toxic and side effects, etc., and achieves superiority Antitumor activity, inhibition of replication, effects of minor toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: c,c,t-[Pt(NH 3 ) 2 Cl 2 (OH)(Aspirin)] preparation
[0019] Dissolve 1 mmol of cisplatin in 5 mL of water, add 10 mmol of hydrogen peroxide, stir the reaction at 50°C for 1.5 h, then stir the reaction at room temperature for 12 h, remove the solvent by rotary evaporation to obtain a solid, wash the solid three times with cold water, and then wash it with ether Washed 3 times and dried in vacuum to obtain the cisplatin oxidation product; it was characterized by electrospray mass spectrometry, the data was MW=332.80, and it could be confirmed that the obtained product was c,c,t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ];
[0020] Take 1 mmol of this substance, dissolve it in 10 mL of dimethyl sulfoxide, add 1 mmol of o-acetylsalicylic anhydride, stir and react at 30°C for 12 hours, freeze-dry to remove the solvent to obtain a solid, add 10-100 mL of acetone to the solid, and centrifuge The resulting precipitate was washed three times with acetone, then three times with ...
Embodiment 2
[0022] Embodiment 2: Preparation of Oxaliplatin (IV)-aspirin compound
[0023] Dissolve 1mmol potassium hexachloroplatinate in 10mL water, add 0.5mmol hydrazine hydrochloride, stir and react at 55°C for at least 3h, then react at 85°C for 6min, place the reaction solution in an ice water bath for 30min, and then perform centrifugation. Take out the supernatant, and remove the solvent by rotary evaporating the supernatant to obtain potassium tetrachloroplatinate;
[0024] Dissolve 1mmol potassium tetrachloroplatinate in 5mL water, add 1mmol trans-1,2-cyclohexanediamine, stir at room temperature for 24h, centrifuge to obtain a solid, dissolve the solid in 5mL water, and then add 2mmol nitric acid Silver, stirred at room temperature for 24 hours, centrifuged, took out the supernatant, then added excess KI, reacted for 12 hours, then centrifuged to take out the supernatant, added equimolar oxalic acid, stirred for 24 hours, centrifuged to separate the solid; First wash 3 times wi...
Embodiment 3
[0028] Example 3: c,c,t-[Pt(NH 3 ) 2 Cl 2 (Aspirin) 2 ] preparation
[0029] Dissolve 1 mmol of cisplatin in 5 mL of water, add 10 mmol of hydrogen peroxide, stir the reaction at 50°C for 1.5 h, then stir the reaction at room temperature for 12 h, remove the solvent by rotary evaporation to obtain a solid, wash the solid three times with cold water, and then wash with ether Washed 3 times and dried in vacuum to obtain the cisplatin oxidation product, which was characterized by electrospray mass spectrometry, the data was MW=332.80, and it could be confirmed that the obtained product was c,c,t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ];
[0030] Take 1 mmol of this substance, dissolve it in 10 mL of dimethyl sulfoxide, add 5 mmol of o-acetylsalicylic anhydride, stir and react at 70 ° C for 12 h, freeze-dry to remove the solvent, add 10-100 mL of acetone to the obtained solid, and centrifuge to separate the precipitate. The precipitate was washed 3 times with acetone, then 3 times with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com