Method for preparing high-purity lithium carbonate from salt lake brine
A technology of high-purity lithium carbonate and salt lake brine, applied in lithium carbonate;/acid carbonate and other directions, can solve the problems of difficult industrial production, poor operability, and high product cost, achieve low cost and reduce production cost , the effect of easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
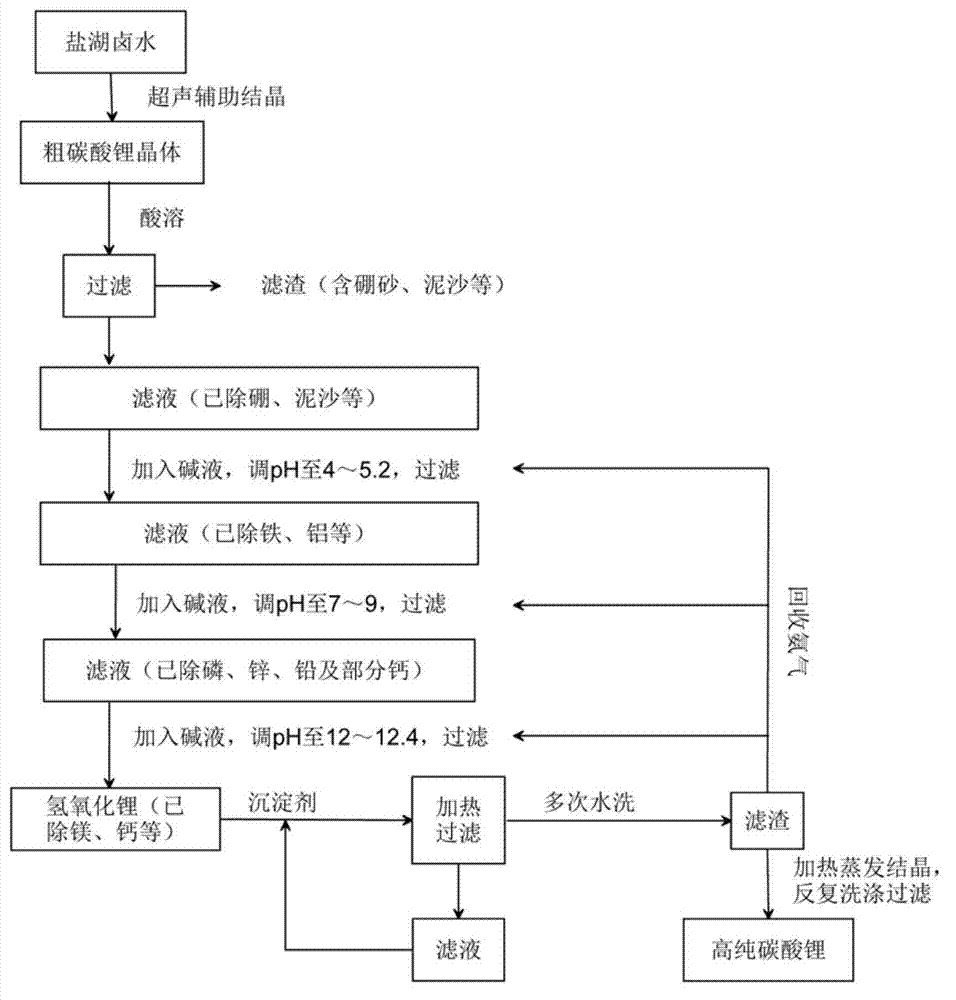
[0032] 1) Introduce Tibetan Zabuye brine into the solar pond for grading and natural evaporation to obtain highly concentrated brine liquid, then introduce the brine liquid into a crystallizer equipped with an ultrasonic oscillator with a frequency of 33kHz, a power of 250W, and 4L, and seed it , using ultrasonic-assisted crystallization, cooling to room temperature after evaporation, and obtaining crude lithium carbonate crystals with a purity of 68-76%;
[0033] 2) the thick lithium carbonate crystal is dissolved in the hydrochloric acid of 20wt% of stoichiometric ratio, namely with Li + Equivalent of hydrochloric acid to dissolve Li in crude lithium carbonate crystals + , then adjust the pH of the solution to 1-2, and filter to remove insoluble matter containing boron, sediment and other substances;
[0034] 3) Add ammonia water to the filtrate obtained in step 2), adjust the pH of the solution to 4.1 and 5.2 respectively, and reach the end point of iron and aluminum preci...
Embodiment 2
[0041] 1) Introduce Tibetan Zabuye brine into the solar pond for grading and natural evaporation to obtain highly concentrated brine liquid, then introduce the brine liquid into a crystallizer equipped with an ultrasonic oscillation device with a frequency of 20kHz, a power of 250W, and 4L, and seed it , using ultrasonic-assisted crystallization, cooling to room temperature after evaporation, performing rapid crystallization, and obtaining crude lithium carbonate crystals;
[0042] 2) the thick lithium carbonate crystal is dissolved in the hydrochloric acid of 20wt% of stoichiometric ratio, namely with Li + equivalent of hydrochloric acid to dissolve Li + , then adjust the pH of the solution to 1-2, and filter to remove insoluble matter containing boron, sediment and other substances;
[0043] 3) Add sodium hydroxide to the filtrate obtained in step 2), adjust the pH of the solution to 4.5, stir and filter, and remove impurities such as iron and aluminum;
[0044] 4) Continu...
Embodiment 3
[0049] The ultrasonic oscillation frequency of the crystallizer is 40kHz; nitric acid is used for the acid solution, ammonia water is used for the alkali solution, and saturated carbon dioxide is used for the precipitating agent; the pH is adjusted to 5.2 in step 3; the pH is adjusted to 7 in step 4; the pH is adjusted to 12 in step 5. Other steps are with embodiment 2.
[0050] The recovery rate of lithium was 77.45%, and the purity of lithium carbonate was 99.9078%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com